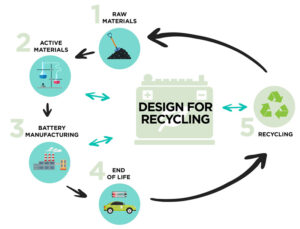
That depends. There is a wide range of regulations for lithium (Li) batteries. Some regulations, like those related to the transport of Li batteries and Li battery packs, have a broader impact than application-focused regulations like those for Li battery packs in electric vehicles (EVs) or industrial systems. This FAQ begins by looking at three…
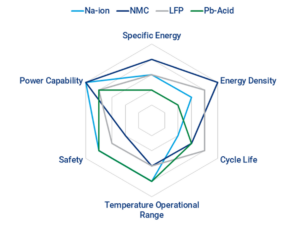
Are Li-ion or Na-ion batteries a more sustainable technology?
That’s a complex and dynamic question without a simple answer. The electrification of everything is expected to lead to post-lithium-ion battery (LIB) technologies like potassium-ion batteries (PIBs), sodium-ion batteries (SIBs), and possibly more exotic chemistries. In the near term, the dominance of LIBs will be almost unassailable. The key word is “almost”. Among the keys…
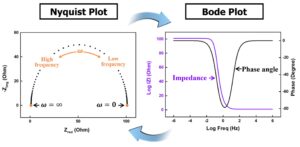
What does electrochemical impedance spectroscopy have to do with Li-ion health?
Electrochemical impedance spectroscopy (EIS) can be used for estimating the power delivery capability and state of health (SoH) of Li-ion batteries. It is important because it has the potential to improve rapid and accurate SoH monitoring of Li-ion batteries and support more sustainable battery storage systems for electric vehicles (EVs) and grid-scale energy storage systems.…
EV basics: Comparing innovative battery chemistries
While lithium-ion battery chemistry dominates the current electric vehicle market, scientists are working to develop innovative battery chemistries that address the known challenges the existing chemistry presents. By Adam Kimmel for Mouser Electronics Sales of electric vehicles (EVs) continue to outpace the overall automotive segment, with EV market shares of 5.2% in Q1 and 5.6%…
Industrial lithium batteries that power the IIoT
By Sol Jacobs, Tadiran Batteries At the heart of the IIoT are lithium battery-operated remote wireless devices that bring digital connectivity to emerging and evolving technologies such as SCADA, process control, industrial robotics, asset tracking, safety systems, environmental monitoring, M2M, AI, and wireless mesh networks, to name a few. Battery-powered remote wireless devices serve to…
Techniques for mitigating thermal runaway in batteries
Thermal runaway happens when a lithium-ion cell, or a small region within a cell, reaches a critical temperature where the materials start to undergo decomposition reactions. These reactions then generate significant additional heat. The decomposition reactions are temperature dependent, increasing exponentially as the temperature increases. Once decomposition starts, a chain reaction causes the battery to…
What are alternatives to Li-ion batteries?
There are many alternatives to Li-ion batteries, including fuel cells, various types of supercapacitors, redox flow batteries, novel Li-based chemistries such as lithium-sulfur (LiS), and more. This FAQ focuses on alternative non-lithium rechargeable battery chemistries, including calcium-ion (Ca-ion), magnesium-ion (Mg-ion), sodium-ion (Na-ion), zinc-ion (Zn-ion), iron-air (Fe-air), and sodium-sulfur (NaS) that can be more readily integrated…
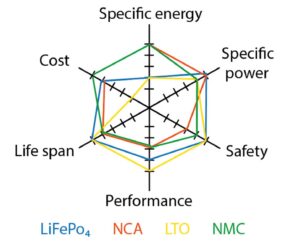
What are six key considerations when choosing a Li-ion battery chemistry?
Designers have choices and tradeoffs when choosing the ideal Li-ion battery chemistry. Batteries affect the cost, lifetime, and usefulness of an application. An optimal energy storage system is critical to ensure the life and performance of an application, so it’s essential to start with the best battery chemistry for the job. This FAQ reviews six…
Surface-mountable Li-ion battery protectors prevent overcharging damage
Littelfuse, Inc. announced the new ITV4030, a series of 22 amp, three-terminal, surface-mountable Li-ion battery protectors. These 4.0 x 3.0 mm devices protect battery packs against overcurrent and overcharging (overvoltage) conditions. The innovative design uses embedded fuse and heater elements that provide fast response and reliable performance to interrupt the charging or discharging circuit before the […]
Li-ion battery protectors prevent overcharging, overcurrent damage
Littelfuse, Inc. announced the new ITV4030, a series of 22 amp, three-terminal, surface-mountable Li-ion battery protectors. These 4.0 x 3.0 mm devices protect battery packs against overcurrent and overcharging (overvoltage) conditions. The innovative design uses embedded fuse and heater elements that provide fast response and reliable performance to interrupt the charging or discharging circuit before the […]