There are several differences between primary and secondary batteries. The most obvious is that primary batteries are single-use devices while secondary batteries can be recharged and used many times, but that’s not the only difference. This FAQ starts with a general overview of the differences between primary and secondary batteries; it will then look at how specific chemistries compare with relation to self-discharge rates, operating temperature capabilities, physical construction, estimation of remaining charge, and power/energy handling.
The electrochemical reactions in primary batteries are not reversible and consume chemicals to produce electricity. Therefore, the larger the size of the primary cell, the more electricity it can produce before it’s depleted. Primary cells are made in a range of standard sizes to power small household appliances and consumer devices such as flashlights and portable radios.
The electrochemical reactions in secondary (rechargeable) batteries are reversible, and the chemical reactants can be restored (recharged) by running a charging current into the battery. In general, rechargeable batteries are more costly to purchase but more economical to use over time compared with primary batteries. For example, the higher costs of rechargeable batteries plus the necessary charger to run a hand-held power tool can be spread over 500 or more recharging cycles, reducing the effective cost per cycle. In this case, it would be much more costly to replace a primary battery to achieve the same time of operation that can be achieved with a rechargeable battery that can be reused 500 times.
Since they cannot be recharged, primary batteries are designed to have very low self-discharge rates. Self-discharge is the loss of charge on a battery that is not being used. Depending on the chemistry and construction, battery self-discharge rates range from a few percentage points per year to 15% per month. Primary cells are on the lower end of that range. However, one of the things that has allowed nickel-metal-hydride (NiMH) rechargeable batteries to continue being produced is new NiMH battery designs with very low self-discharge rates that are comparable with many inexpensive primary cells. And, while they cost more than primary batteries, NiMH batteries are among the least-expensive rechargeable chemistry, making them attractive in a variety of use cases.
A performance advantage of rechargeable batteries such as NiMH and Li-ion is their ability to support higher power levels without damage. NiMH and Li-ion cells have much lower internal resistance and do not experience the large capacity loss that primary batteries suffer when supporting higher current demands.
Primary batteries
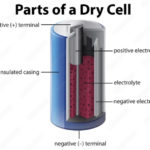
Zinc-carbon “dry cells” are common primary batteries. The zinc container acts as both a package to hold the other active chemicals and as the negative electrode. The positive electrode is a carbon rod in the center of the cell. It is surrounded by a paste containing manganese (IV) oxide, zinc chloride, ammonium chloride, carbon powder, and a small amount of water. All zinc-carbon dry cells have an initial voltage of about 1.5V, and it decreases as the battery is discharged. Of course, larger batteries can deliver more electricity. One disadvantage of these batteries is that as the zinc container oxidizes, its contents can eventually leak. So, zinc-carbon batteries should only be in devices as they are being used and not stored in any device for an extended period.
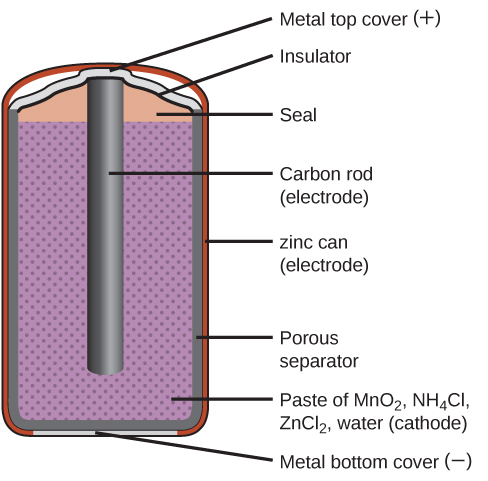
Zinc-carbon dry cell internal construction. (Image: LibreTexts)
Alkaline primary batteries were developed to be improved replacements for zinc-carbon dry cells. An alkaline can deliver up to 5X the energy of a similarly-sized zinc-carbon battery. Like zinc-carbons, alkaline batteries are prone to leaking, in this case, potassium hydroxide, so these should also be removed from devices for long-term storage. Unlike zinc-carbons, which are never rechargeable, some alkalizes can be recharged. However, attempting to recharge an alkaline battery that is not specially designed to be rechargeable can rapture the battery, causing leakage of the potassium hydroxide electrolyte. While rechargeable alkalizes are relatively inexpensive, they can only be recharged relatively few times compared with other chemistries like NiMH or Li-ion.
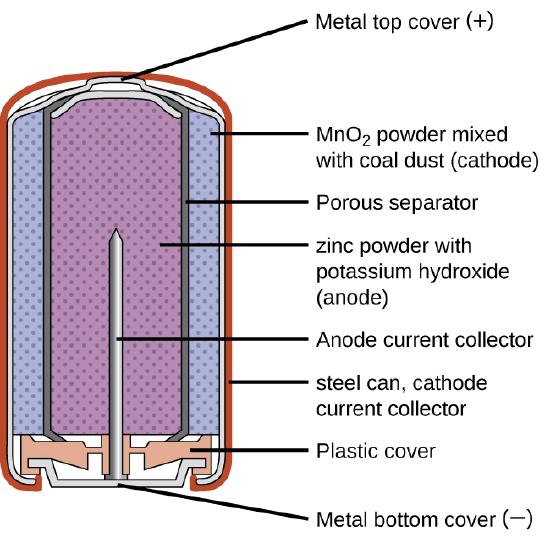
Alkaline primary batteries were developed as a higher-performance alternative for zinc-carbon batteries. (Image: LibreTexts)
Several other common primary battery chemistries have been developed for specific performance levels, including zinc chloride as a “heavy-duty” alternative to zinc carbon. In addition, there are additional chemistries based on zinc, several primary battery types use lithium chemistries, and there are high drain mercury oxide cells and silver oxide button cells.
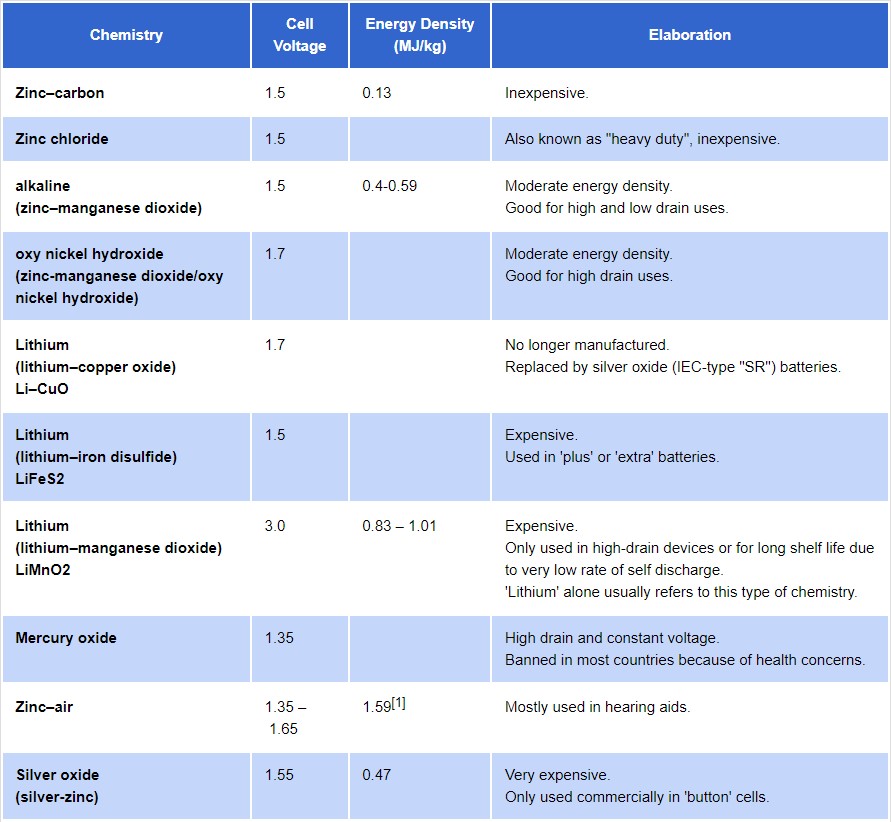
Summary of common primary battery chemistries. (Image: Epec, LLC)
Rechargeable batteries
NiMH or Li-Ion rechargeable cells, or lithium primary cells, are generally better suited to power electronic devices than alkaline batteries. This is because each of these three rechargeable chemistries produce a more consistent voltage compared with alkaline.
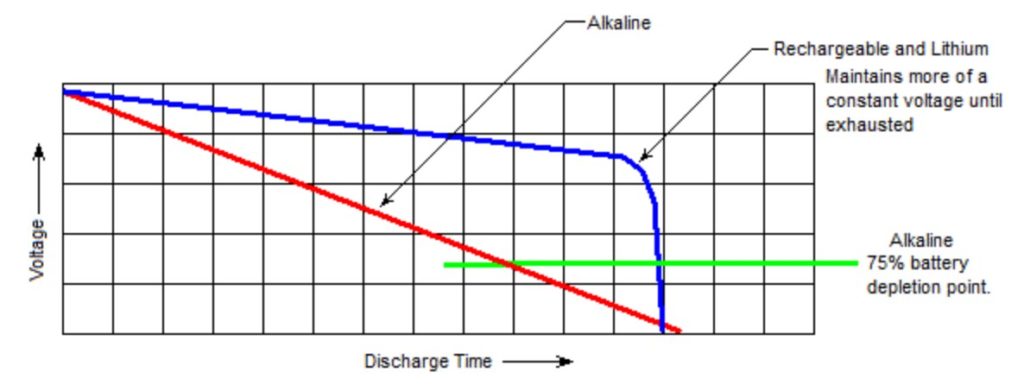
Alkaline vs. lithium discharge voltage curves. (Image: Torchspot)
There are a wide variety of rechargeable lithium chemistries (the six most common are detailed below). However, in their simplest embodiment, a rechargeable lithium battery consists of a graphite anode, a lithium metal oxide cathode, and an electrolyte of a lithium salt and an organic solvent. When used in rechargeable batteries, lithium has two significant advantages: it has a large electrode potential voltage, resulting in batteries with relatively high voltages that can support higher power levels. In addition, it is the metal with the lowest density, which results in lower battery weights.
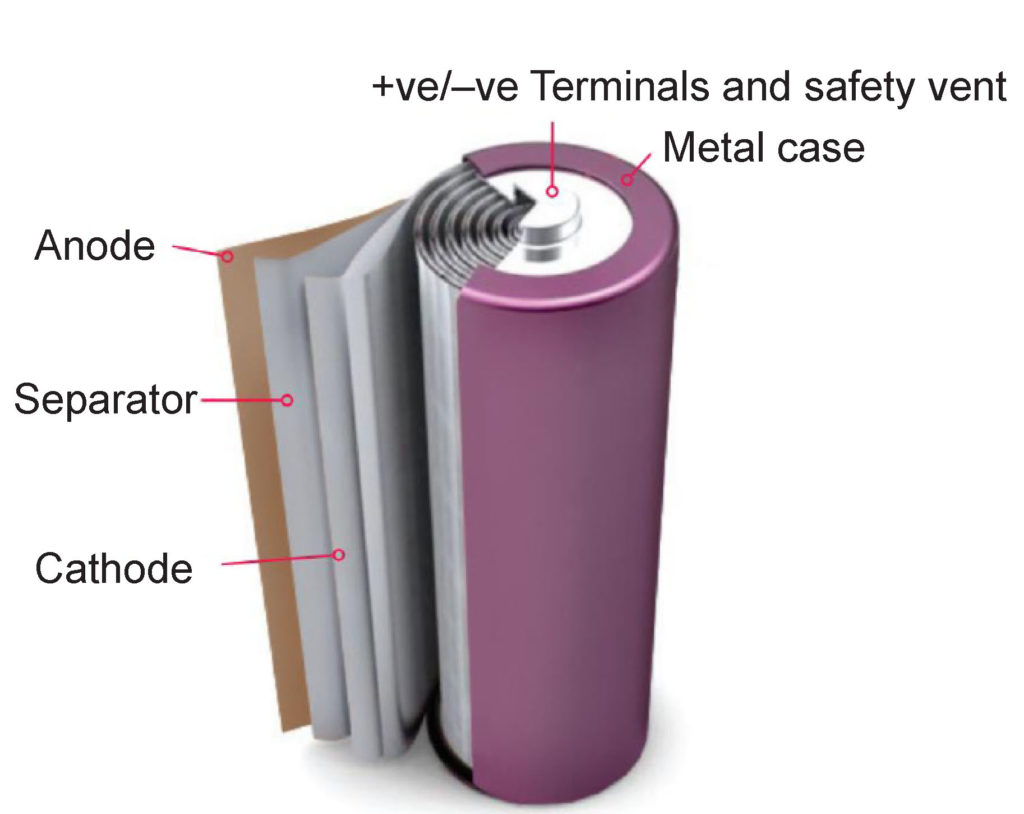
Cylindrical battery construction is commonly used for nickel and lithium rechargeable chemistries. (Image: Johnson Matthey)
Nickel and lithium cells are often produced with a cylindrical shape, shown above, which consists of a long assembly of the positive electrode, separator, negative electrode, and separator layers rolled into a single spool. While these cylindrical cells can be produced faster than cells with stacked structures, they can suffer from large internal radial temperature gradients at high discharge currents.
Li-ion batteries are also offered in soft pouches, or rigid plastic cases called prismatic formats. The absence of a case gives pouch cells the highest gravimetric energy density. Soft pouches are often used in portable consumer electronics devices. Rigid plastic cases with large threaded terminals are more rugged and can be found in larger formats for electric vehicles, renewable energy storage, and other large-scale applications.
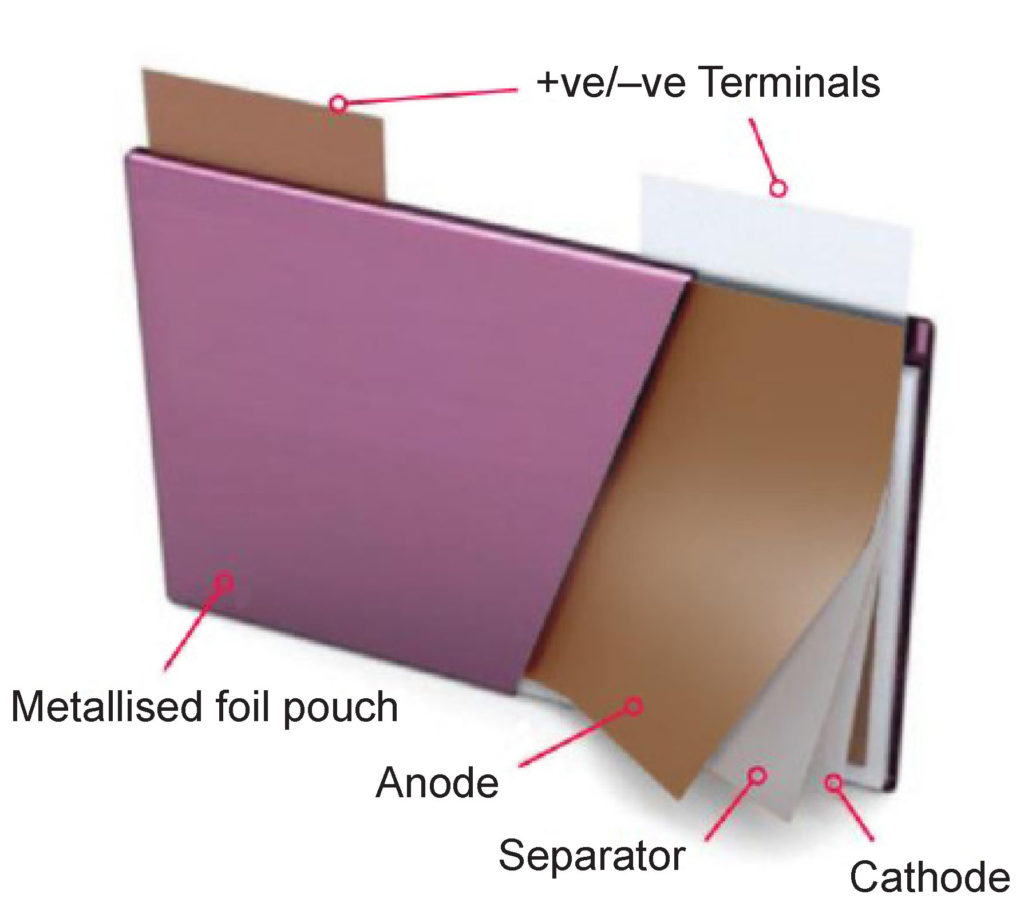
Pouch cell internal construction. (Image: Johnson Matthey)
The following section compares the performance of lead-acid, NiCd, and NiMH batteries with the six most common rechargeable lithium chemistries:
Lead-acid batteries are primarily used in motive power applications such as material handling equipment and automobiles. And they are used as energy storage devices in uninterruptible power systems and some renewable energy and telecommunications applications. They are moderately expensive, have low gravimetric energy levels, and moderate self-discharge rates.
NiCd is rarely used due to the environmental hazards caused by cadmium; they are largely prohibited in Europe and other regions.
NiMH can be inexpensive alternatives to primary batteries in some electronics applications; they are generally unsuitable for high drain applications. Newer chemistries have very low self-discharge rates but a 25% lower energy density than older formulations.
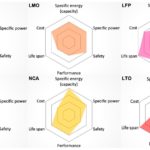
LTO (Lithium Titanate) is expensive but offers superior safety and low-temperature capabilities than other rechargeable lithium chemistries. They have a cycle life of up to 7,000 cycles and can be quickly charged and discharged (they support high power levels)
LFP (Lithium Iron Phosphate) has low internal resistance and can support relatively high discharge currents, except at cold temperatures. However, they do not generally support quick charging. Self-discharge can be more of an issue compared with some other rechargeable lithium chemistries. LFP tends to be safe with a good lifetime, but elevated storage temperatures can shorten service life.
LMO (Lithium Manganese Oxide) batteries have high thermal stability and enhanced safety, but cycle and calendar lives are limited. Low internal cell resistance enables fast charging and high-current discharging.
NMC (Lithium Nickel Manganese Cobalt Oxide) is available in several different formulations that can deliver high specific energy or high specific power. High cell voltage (3.7V) supports higher energy density with lower cost and good cycle life.
LCO (Lithium Cobalt Oxide) High specific energy, but limited specific power. Lower thermal stability and shorter lifetime. It cannot support high current levels.
NCA (lithium nickel cobalt aluminum oxide) is not commonly found in consumer devices but is becoming increasingly important in electric vehicle power trains and grid storage. NCA batteries provide a high-energy option with a good lifespan. However, they are not as safe as other lithium-ion battery types and are quite costly.
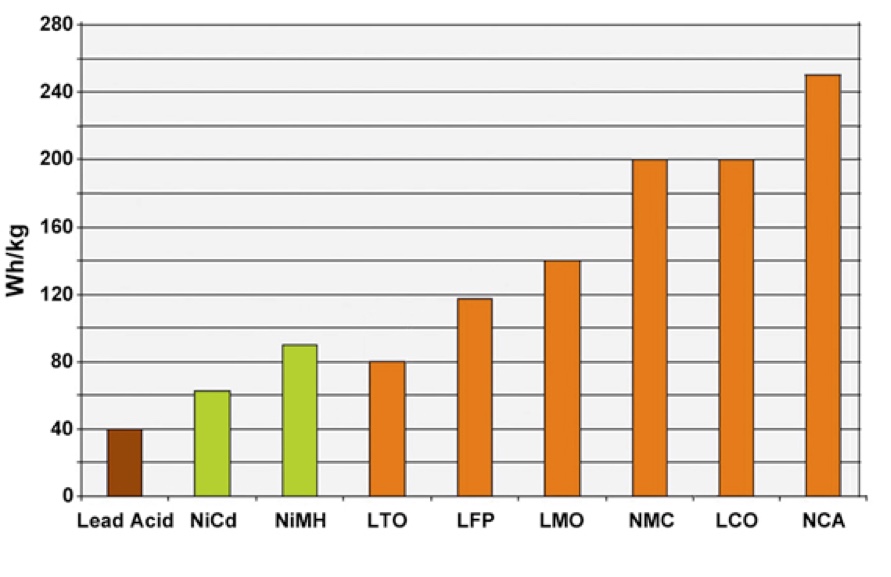
Lithium battery chemistries deliver higher gravimetric energy levels than other rechargeable batteries (Image: MIUTA Electric Co)
Summary
As shown, there are a wide variety of primary and secondary battery choices available to designers to meet the performance needs of specific applications. For rechargeable lithium alone, there are six common chemistries to chose from. The optimal selection depends on a complex matrix of considerations, including self-discharge rates, operating temperature capabilities, fast charging ability, physical construction, estimation of remaining charge, safety concerns, and power/energy handling capabilities.
References
Automotive Lithium-Ion Batteries, Johnson Matthey
Battery chemistry, Epec, LLC
Batteries and Fuel Cells, LibreTexts
Primary cell, Wikipedia


Tell Us What You Think!