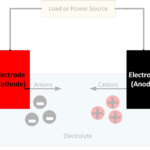
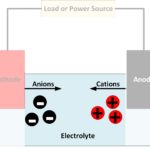
Wet cell batteries have a pool of liquid electrolytes; they generate gases meaning they require venting and must be kept upright to avoid leakage. Dry cell batteries use paste electrolytes, which contain enough liquid for good electrical conductivity, but are stable enough not to leak when turned upside down.
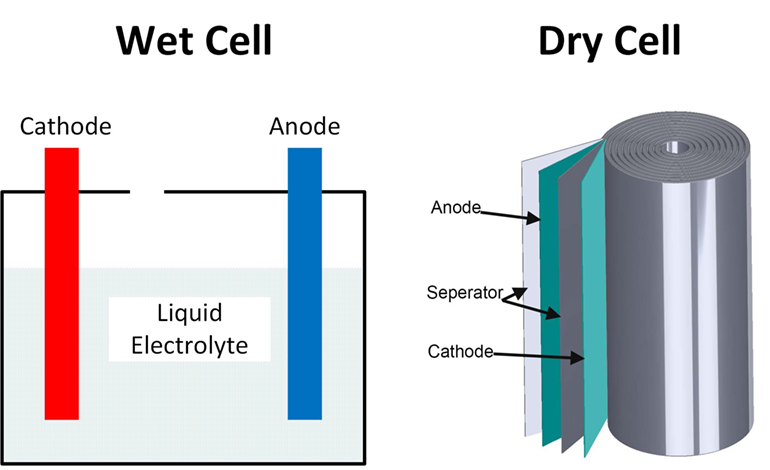 The first batteries were wet cells constructed in labs using open glass containers. Lead-acid wet cell batteries are still commonly used as car batteries and for backup power in buildings. In this type of wet cell battery, both electrodes are lead plates, which are suspended in a tank of sulphuric acid, acting as the electrolyte. Wet cell batteries enable high power outputs, and rapid discharge as the free flow of electrolytes facilitates high currents. They are also cheaper and easier to manufacture, with good resistance to overcharging. However, they are large, heavy, and can leak corrosive chemicals, making them difficult and dangerous to handle.
The first batteries were wet cells constructed in labs using open glass containers. Lead-acid wet cell batteries are still commonly used as car batteries and for backup power in buildings. In this type of wet cell battery, both electrodes are lead plates, which are suspended in a tank of sulphuric acid, acting as the electrolyte. Wet cell batteries enable high power outputs, and rapid discharge as the free flow of electrolytes facilitates high currents. They are also cheaper and easier to manufacture, with good resistance to overcharging. However, they are large, heavy, and can leak corrosive chemicals, making them difficult and dangerous to handle.
Absorbed glass mat (AGM) and gel batteries are valve-regulated, lead-acid batteries that blur the line between wet and dry cells. The sulphuric acid is stabilized in these batteries by being absorbed in a glass fiber mat or silica gel. This means they can be inverted without spilling, although they still require vent gas through a valve.
Most types of batteries in current usage are dry cell. This includes both single-use primary cells and rechargeable secondary cells. Examples of dry cell primary cells include zinc-carbon and alkaline cells. Examples of secondary cells include lithium-ion and nickel-metal hydride cells Dry cell batteries are more convenient for mobile applications and now often offer higher performance. They are small, light, and do not leak chemicals, making them easy to handle. However, they tend to be more expensive and difficult to manufacture and are less resistant to overcharging.
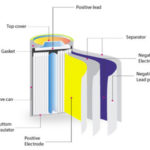
Lithium-ion batteries are a particularly important type of dry cell battery. They use an aqueous lithium salt solution as the electrolyte, applied as a thin layer onto separator sheets sandwiched between the cathode and anode materials, which are also coated onto thin sheets. Typically this stack of sheets is rolled up to form a cylindrical battery cell.


Great article, one question; I have a YYD Robo electric scooter with a 350w, motor, with a 36v, 7.5ah lithium battery. Is this a dry cell battery? They won’t let me ride the bus with the scooter claiming it doesn’t have a dry cell battery.
I have a 1984 Yamaha Venture 1200, and I changed the battery without thinking it though. I put a dry cell battery in where a wet cell should have went. So there is a wire that went into the wet cell, is there any way of bypassing that! So I can just keep the dry cell in it!
As the article implies dry cell are better than wet cells the limit of a wet cell charging system is the lack of overcharge & cut off circuit to trip off charging when battery gets fully charge
Is a 120 amp lithium caravan battery classed as a dry or wet not sure what to set up
The question is which is better Wet or Dry cell.
Please lets have the truth so as to make informed decision.
Though you tried to highlight the advantages of the dry cell, is it the best one can go for?