Flow batteries have emerged as a commercial alternative that makes economic sense for certain application profiles. Additional development is still needed for flow batteries to be a mainstream energy storage technology. There are probably hundreds of research efforts worldwide. The following is a sampling of key areas of focus for flow battery research and development. Work is underway to improve current flow battery technologies as well as to develop new alternatives.
For example, to date, the cell area for practical use in Vanadium flow batteries has been around 40 square centimeters. Research is underway at thyssenkrupp AG and other organizations to achieve a cell size of over 2.50 square meters. That would make it possible to build industrial storage systems with an initial output of 20 megawatts and a capacity of 200 megawatt hours.
Better bromine batteries
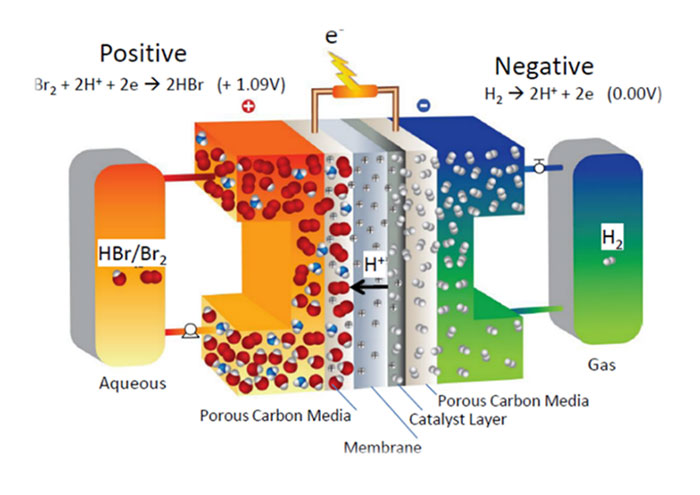
Hydrogen, Zinc and Bromine are abundantly available at low costs on a global scale. The supply is not restricted to geographical availability, and cannot be dominated by a small group of suppliers (unlike Lithium, Cobalt and Vanadium). As a result, several efforts are underway to develop Zinc-Bromine and Hydrogen-Bromine batteries.
Gelion has developed a unique platform technology that can be specifically adapted to the performance requirements of the battery. Gelion does this by combining the advantages of ionic liquids and innovative additives, using Zinc-Bromine chemistry in combination with advanced electrolytes that can be all-liquid, liquid/ionogel or all-ionogel.
Gelion ENDURE battery cell (Image: Gelion)
As a result, Gelion has transformed the Zinc Bromide redox flow-battery technology into a more conventional stationary architecture. Instead of a pumped battery system with tanks and moving parts, the chemistry can be a self-contained block, like a Lead acid or alkaline cell. This results in a consumer-friendly package that is much more economical, scalable and maintenance friendly, while retaining all the benefits of the Zinc Bromide technology.
The EU recently awarded €4 Million to the MELODY consortium, to develop low cost, innovative batteries for large-scale energy storage based on Hydrogen-Bromine chemistry, as part of the Horizon 2020 program ‘Advanced Redox Flow Batteries for stationary energy storage’. The MELODY consortium consists of small & medium enterprises (Elestor, PV3 Technologies, Vertech), industry (Shell) and academic leaders (TU Delft, Technion, University of Exeter, ETH Zurich), with coordination support provided by Hezelburcht. The collaborative project began in January 2020, and will run for 4 years, leading to a pilot facility that demonstrates the practical application.
Elestor Hydrogen-Bromine redox-flow battery that is part of the EU’s MELODY project (Image: Elestor)
MELODY aims to develop a sustainable redox flow battery technology that can effectively reduce the costs of electricity storage to support large-scale, global deployment. Mass market introduction of redox flow batteries has been hampered by various factors – material cost, limited catalyst lifetime, membrane costs, system complexity and safety issues. The development of an economically viable and sustainable Hydrogen-Bromine redox-flow battery (RFB) storage systems is anticipated. To overcome these challenges, MELODY employs a unique triple cost reduction strategy, specifically:
- A membrane-less flow battery concept;
- The use of abundant hydrogen and bromine;
- A simplified system design.
Organic flow batteries
JenaBatteries has developed this technology based on a so-called redox flow battery (RFB) with organic materials, thus has the world’s first commercially available technology of this kind. Two liquid organic electrolytes separated by a membrane and stored in separate tanks store the current. BASF will supply one of the two electrolytes as part of the collaboration. This battery material is based on an amine, a chemical intermediate that the company can produce on an industrial scale.
Metal-free redox flow battery (Image: JenaBatteries)
Amines are organic compounds which contain and are often actually based on one or more atoms of nitrogen. Structurally amines resemble ammonia in that the nitrogen can bond up to three hydrogens, but amines also have additional properties based on their carbon connectivity. In an amine, one or more of the hydrogen atoms from ammonia are replaced by organic substituents like alkyl (alkane chain) and aryl (aromatic ring) groups.
The JenaBatteries’ RFBs store electrical energy in organic compounds. The two reaction partners are present in dissolved form and circulate in two separate circuits. The ion exchange between the two energy-storing electrolytes takes place through a membrane in the galvanic cell. Here the chemical reduction or oxidation of the dissolved substances takes place. Electrical energy is absorbed during charging and released during discharging.
Researchers at Harvard University are developing organic redox flow batteries based on quinone-bearing aqueous electrolytes. The lifetime of these batteries is currently limited by quinone stability. The researchers recently showed that the decomposition can be substantially mitigated. It is expected that the mitigation strategy could be a step toward realizing renewable electricity storage through long-lived organic flow batteries.
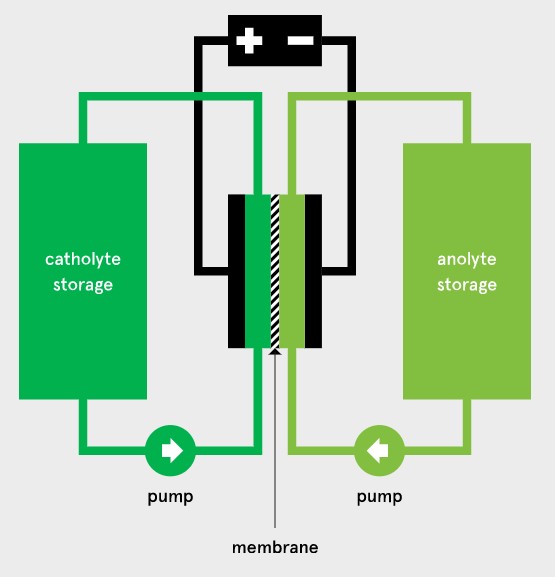
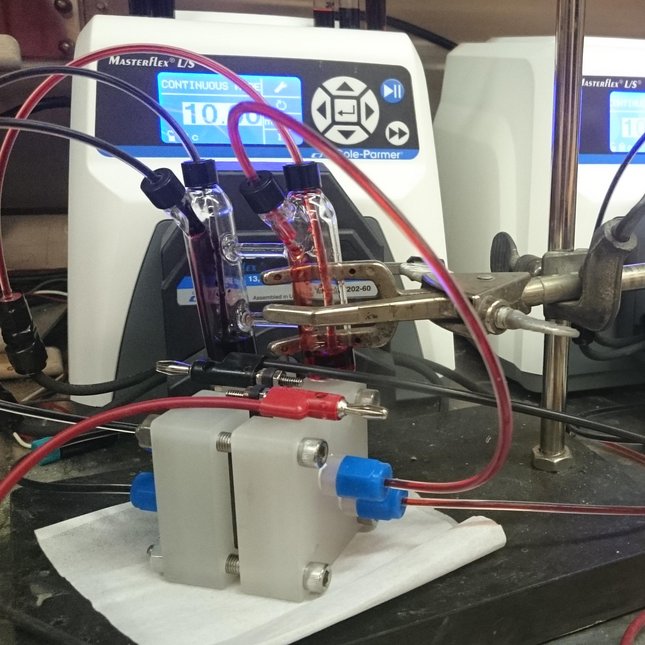
Eindhoven University of Technology (TU/e) researchers are working toward non-aqueous organic redox flow batteries from abundant all-carbon based materials can provide a sustainable solution. In a redox flow battery (RFB), the redox active species are dissolved or suspended in a solvent with supporting electrolyte forming an anolyte and catholyte. These solutions flow through a reactor where they are passed over porous carbon electrodes that mediate electron transfer reactions to charge or discharge the solutions. The two half-cells in the reactor are separated by a selective permeable membrane, which allows for a compensating ion flux opposing the electrical flow of the external circuit.
Non-aqueous organic redox flow battery test cell (Image: Eindhoven University of Technology)
Typically, the membrane has the highest electrical resistance of the whole system and therefore has a large impact on the energy efficiency of the battery, especially at high charging or discharging rates. Its role is twofold as it must keep the anolyte and catholyte molecules separated while being highly conductive towards the supporting electrolyte.
Additionally, the membrane must have sufficient mechanical stability to withstand the shear forces of the electrolyte flow and resist swelling from the solvent. Membranes that combine all of these properties are therefore very scarce. Together with collaborators, the researchers at TU/e are developing and testing tailored membranes for this application.
That concludes this three-part FAQ series. Part one discussed, “Flow Batteries – How Does That Work?” and part two focused on “Flow Batteries – What Can You Use Them For?”
References
Hydrogen bromine battery, Wikipedia
Redox flow batteries: Energy storage systems for renewables, thyssenkrupp AG
TU Delft leads new European project on membrane-less redox flow batteries, TU Delft