By Ashok Lahiri, Enovix Corporation
The first lithium-ion (Li-ion) battery, developed and commercialized by Sony Corporation in 1991, provided a step-change increase in energy density for its handheld camcorder — a harbinger of the many power-hungry portable electronic devices to come. Without this battery innovation, the brick-size cell phone of the 1980s would never have evolved into today’s sleek, sophisticated smartphone.
Today, next-generation mobile devices — such as smartwatches and smartphones with performance enhancements including 5G cellular and on-device AI — need higher energy density batteries in sleek form factors. To fully realize the promise of next-generation mobile devices, another step-change increase in battery energy density and capacity is needed.
Conventional Li-ion cell architecture
As Sony prepared to produce the first Li-ion battery, manufacturing engineers noticed a surplus of magnetic tape production equipment due to a shift in the market for recorded music from audio cassettes to compact disks. Magnetic recording tape was made on production lines that coated a plastic film with a magnetic slurry, dried it, cut it into long strips, and rolled them up. Sony repurposed the equipment to coat chemical slurries onto metal foil current collectors, dry them, and cut them into electrode sheets.
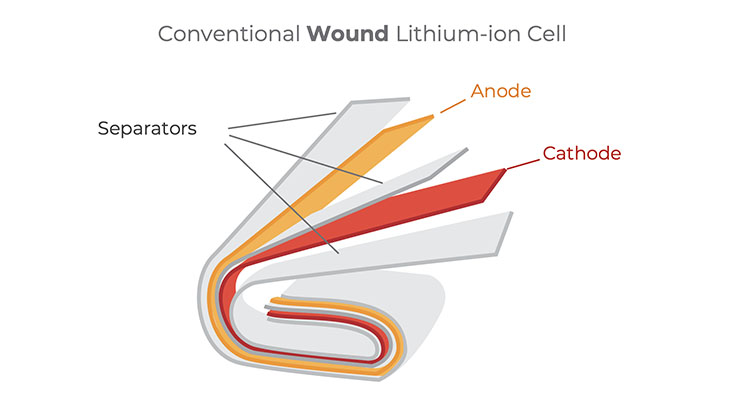
The illustration below shows the resulting architecture of a conventional “jelly roll” wound Li-ion cell. Long electrode sheets — a cathode (positive electrode) and an anode (negative electrode) — are wound together, with a permeable polymer separator between them, and packaged in a metal case (shown) or polymer laminate pouch. Electrolyte, a chemical medium (usually containing a lithium salt in an organic solvent mixture), is added to enable the movement of ions through the separator between the anode and cathode.
Most Li-ion batteries today use a graphite anode, carbon in a crystalline form. A graphite anode absorbs lithium ions when charging and releases them back into the electrolyte when the battery discharges (i.e., powering a device). At the anode, lithium (Li) atoms intercalate into spaces between graphite (C) layers and combine at a one-to-six (1:6) ratio (LiC6). This gives graphite a theoretical specific capacity of about 372 milliamp-hours per gram (mAh/g).
The promise and challenge of a silicon anode
Researchers have long known that a silicon anode could significantly increase the energy density of a conventional Li-ion battery. Silicon (Si) is an attractive anode material because it forms a Li15Si4 alloy. Its increased Li-to-Si bonding ratio gives silicon a theoretical specific capacity of about 3,579 mAh/g, over nine times that of graphite.
Unfortunately, a predominately silicon anode in conventional Li-ion cell architecture creates technical problems. Because lithium atoms slip into the spaces between graphite layers, minimal anode swelling occurs during charging cycles (typically <10%). But lithium atoms form a Li15Si4 lithium-silicon alloy in a silicon anode rather than intercalate between layers. While this alloying process results in a greater ability to store lithium, the increased amount of lithium in the anode can create large volume changes that can result in high pressures within the battery.
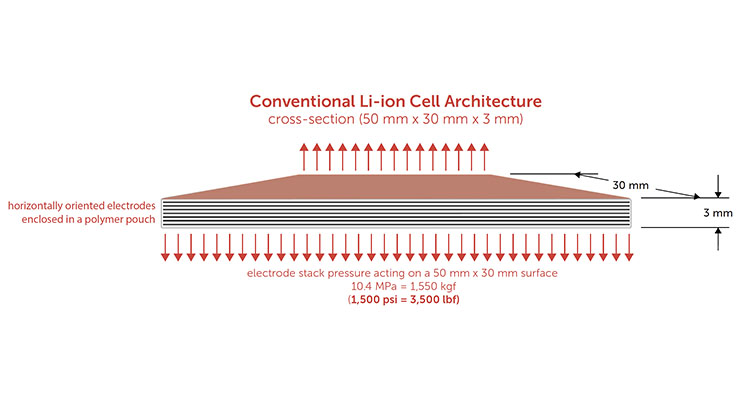
As illustrated below, if a 100% active silicon anode (i.e., silicon as the only active lithium cycling material) were used in a conventional battery architecture, the pressure of anode swelling would act on the large (50 mm x 30 mm) face of the battery (red), requiring a force as large as 1,550 kgf or 3,500 lbf to contain swelling during cycling. The required force is analogous to a car standing on a cell phone-sized battery.
Without a large counterforce to prevent further swelling, the anode can continue to expand further with each charge/discharge cycle. During repeated charge-discharge cycles, silicon particles can become electrically disconnected and crack. In addition, when silicon particles become disconnected from the electrode, they can no longer accept lithium, and neighboring particles must absorb the excess, causing overcharging and further opportunities for physical damage.
Collectively, these problems result in an unacceptably low battery cycle life (i.e., the number of times a battery can be fully charged and discharged during its life before reaching 80% capacity). This makes a 100% active silicon anode in conventional battery cell architecture impractical for most mobile device applications.
Changing cell architecture to harness silicon
Companies are taking different approaches to achieve the benefits of a silicon anode. Some simply blend small amounts of silicon (typically 3% – 7%) with graphite in conventional Li-ion cell architecture. At the other end of the spectrum, companies are developing exotic materials such as silicon nanowires and nanostructured cores containing silicon within a carbonized scaffolding matrix. One company, Enovix Corporation, has taken a very different approach to developing a proprietary cell architecture to harness the benefits of 100% active silicon.
As illustrated above, rather than long, wound electrodes that run parallel to the face of the battery, Enovix cells have many small laser-patterned electrodes that are orthogonal to the largest face of the battery. When silicon anodes are stacked in this cell architecture, they do not face the largest side of the battery. Instead, as illustrated below, the anodes face a short (3 mm x 30 mm) side of the battery (blue). Because the anode faces a smaller surface area, this same 10.4 MPa or 1,500 psi pressure requires a force of only 94 kgf or 210 lbf to contain expansion. A thin, stainless-steel constraint system surrounds the cell, providing sufficient force to prevent swelling of the battery.
Enovix cell architecture
This novel approach to cell architecture can manage the first charge expansion, further swelling during repeated charge/discharge cycles, and shortened cycle life associated with a 100% active silicon anode in conventional cell architecture. The result is a battery that delivers an increase in energy density from 25% to over 65%, depending on cell size, with over 500 full depth of discharge cycle life. With this battery innovation, consumers can fully realize the promise of next-generation mobile devices.
About the Author
 Ashok Lahiri has served as a technical advisor to Enovix since February 2023. Before this role, he was CTO of Enovix since co-founding the company in 2007, where he was the lead architect of the Enovix Silicon Lithium-ion Rechargeable Battery, responsible for the design and implementation of the patented cell architecture and high-capacity silicon anode. Previously, he served in engineering and management positions with FormFactor (semiconductor wafer testing) and IBM (high-density hard-disk drives). Ashok earned a B.S. with honors in chemical engineering from U.C. Berkeley. He has authored or co-authored over 20 patents or applications in battery technology, 3D architecture, and electrochemistry.
Ashok Lahiri has served as a technical advisor to Enovix since February 2023. Before this role, he was CTO of Enovix since co-founding the company in 2007, where he was the lead architect of the Enovix Silicon Lithium-ion Rechargeable Battery, responsible for the design and implementation of the patented cell architecture and high-capacity silicon anode. Previously, he served in engineering and management positions with FormFactor (semiconductor wafer testing) and IBM (high-density hard-disk drives). Ashok earned a B.S. with honors in chemical engineering from U.C. Berkeley. He has authored or co-authored over 20 patents or applications in battery technology, 3D architecture, and electrochemistry.



Tell Us What You Think!