By Sol Jacobs, Tadiran Batteries
At the heart of the IIoT are lithium battery-operated remote wireless devices that bring digital connectivity to emerging and evolving technologies such as SCADA, process control, industrial robotics, asset tracking, safety systems, environmental monitoring, M2M, AI, and wireless mesh networks, to name a few.
Battery-powered remote wireless devices serve to economically capture, exchange, store, analyze, and apply data intelligently. These devices form the backbone for Industry 4.0 by eliminating the cost and complexity of hard-wiring. With numerous battery chemistries to choose from, the specifying process often revolves around five main criteria:
Determining the energy demand
Identifying the best battery chemistry
Understanding the importance low self-discharge
Adapting for high pulse requirements
Comparing seemingly similar batteries
A remote wireless device is only as reliable as its battery, so design engineers must choose wisely based on a number of criteria, including: the amount of energy consumed in ‘active’ mode (including the size, duration, and frequency of pulses); the amount of energy consumed while in ‘standby’ mode (the base current); storage time (as normal self-discharge during storage diminishes capacity); thermal environments (including storage and in-field operation); equipment cut-off voltage (As battery capacity is exhausted, or in extreme temperatures, voltage can drop to a point too low for the sensor to operate.), and, most critically, the annual self-discharge rate (which often exceeds the amount of energy consumed while the device operates).
Sometimes wireless devices are easily accessible for battery replacement and operate within relatively mild temperatures. Here, the approach taken could involve an inexpensive consumer-grade alkaline or lithium battery. However, an industrial grade lithium battery is typically required if the application involves a long-term deployment in a hard-to-access location or extreme environment.

AI-enabled electronics collars allow ranchers to remotely track their cattle herds by providing behavioral information and alerts using an ultra-low-power LoRaWAN network. Selective members of the herd carry solar-powered communicators that form a wireless mesh network covering the entire herd. Here Tadiran TLI Series rechargeable Li-ion batteries can withstand extreme temperatures, offer up to 20-year operating lives and 5,000 full recharge cycles, and can generate the high pulses required to power wireless communications.
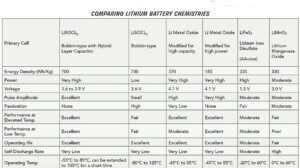
To conserve energy and extend battery life, low-power remote wireless devices must operate mainly in a standby state, drawing micro-amps of average current with high pulses in the multiple-ampere range. These low-power applications are predominantly candidates for bobbin-type lithium thionyl chloride (LiSOCl2) batteries, which feature a high capacity and energy density, an extended temperature range, and an exceptionally low annual self-discharge rate.
There are also certain applications that draw higher average currents measurable in milliamps with pulses that can be in the multi-ampere range. This kind of power dissipation can sap enough average energy to prematurely exhaust a primary (non-rechargeable) battery. As a result, these niche applications are often better suited for some form of energy harvesting device working in conjunction with an industrial grade Lithium-ion (Li-ion) battery that stores the harvested energy.

Ayyeka AI-enabled smart sensors are used to monitor and better maintain hard infrastructure used in solid waste and wastewater management, public utilities, transportation, energy exploration and distribution, smart cities, environmental monitoring, and more. Powered by Tadiran bobbin-type LiSOCl2 batteries, these remote wireless devices permit two-way communications to maximize operational efficiency, detect unusual events, enable predictive maintenance and repairs, and help counter cyber security threats.
Numerous primary (non-rechargeable) lithium battery chemistries are available, each offering their own advantages and disadvantages. At one end of the spectrum are inexpensive alkaline batteries. These cells deliver high continuous energy but suffer from a high self-discharge rate (which limits battery life), low capacity and energy density (which adds size and bulk), and an inability to operate in extreme temperatures due to water-based constituents. At the other end of the spectrum are industrial grade lithium batteries.
As the lightest non-gaseous metal, lithium features an intrinsic negative potential that exceeds all other metals, delivering the highest specific energy (energy per unit weight), highest energy density (energy per unit volume), and higher voltage (open circuit voltage, OCV) ranging from 2.7 to 3.6 V. Lithium battery chemistries are also non-aqueous and are thus less likely to freeze in extreme cold.
Bobbin-type LiSOCl2 chemistry is overwhelmingly preferred for long-term deployments, delivering the highest capacity and highest energy density, enduring the most extreme temperatures (-80 to +125°C), and featuring an annual self-discharge rate as low as 0.7% per year for certain cells. A self-discharge rate this low enables up to 40-year battery life. Bobbin-typeLiSOCl2 batteries were specifically designed for use with low-power communication protocols such as WirelessHART, ZigBee, and LoRA. This chemistry offers the following benefits:
Higher reliability – useful for remote locations where battery replacement is difficult or impossible and there’s a need for highly reliable connectivity.
Long operating life – because the battery’s self-discharge rate often exceeds actual energy use, high initial capacity and a low annual self-discharge rate are critical.
The widest temperature range – bobbin-type LiSOCl2 cells can be modified to work reliably in extreme temperatures (-80 to 125°C).
Smaller size – their higher energy density can permit the use of batteries that take up less space.
Higher voltage – delivering 3.6 V, they could allow for the use of fewer cells.
Lower lifetime cost – often a critical consideration because the labor and logistical expenses for replacing a battery will far exceed its cost.
Low battery self-discharge
All batteries experience some amount of self-discharge as chemical reactions draw current even while the cell is not in use or is disconnected. Self-discharge can be minimized by controlling the passivation effect, where a thin film of lithium chloride (LiCl) forms on the surface of the lithium anode to separate it from the electrode. This film reduces the chemical reactions that lead to higher self-discharge. Whenever a current load is drawn from the cell, the battery experiences initial high resistance and a temporary drop in voltage until the discharge reaction begins to dissipate the passivation layer. This process repeats continually each time a load is applied.
The level of passivation can vary based on numerous variables, including the cell current discharge capacity, the length of storage, storage temperature, discharge temperature, and prior discharge conditions, as partially discharging a cell and then removing the load increases the level of passivation over time. Passivation may be helpful for reducing self-discharge, but too much of it can be problematic if it overly restricts energy flow.
Bobbin-type LiSOCl2 cells range considerably in terms of their ability to harness the passivation effect. For example, the highest quality LiSOCl2 cells can exhibit a self-discharge rate as low as 0.7% per year, thus retaining nearly 70% of their original capacity after 40 years. Conversely, lower-quality LiSOCl2 cells can have a self-discharge rate as high as 3% per year, exhausting nearly 30% of their available capacity every 10 years.
To support two-way wireless communications, numerous low-power devices require periodic high pulses of up to 15 A. Standard bobbin-type LiSOCl2 cells can’t deliver high pulses because of their low-rate design. The solution is the addition of a patented hybrid layer capacitor (HLC). With this hybrid approach the standard bobbin-type LiSOCl2 cell delivers nominal background current during ‘standby’ mode while the HLC delivers high pulses to power data communications. The HLC also experiences a unique end-of-life voltage plateau that can be interpreted to generate ‘low battery’ status alerts.
Supercapacitors sometimes serve as power sources for consumer products, but they are poorly adapted to industrial applications. They have serious limitations that include short-duration power; linear discharge qualities that do not allow for the use of all available energy; low capacity; low energy density; and self-discharge rates up to 60% per year. Supercapacitors linked in series also require the use of expensive cell-balancing circuits that add bulk and drain additional current that can further shorten their operating life.
With any long-term deployment it can be useful to have the battery last for the entire lifetime of the device, thus eliminating the need for battery change-outs. Unfortunately, it can be extremely difficult to distinguish a higher-quality cell from a poorer quality competitor because cell capacity losses caused by self-discharge may not reveal themselves for years. Additionally, the theoretical models and algorithms used to calculate battery life expectancy tend to be highly unreliable; they often underestimate the passivation effect as well as long-term exposure to extreme temperatures.
These uncertainties force designers to perform careful due diligence when evaluating batteries for any long-term deployment. As part of the specifying process, competing battery suppliers should be required to provide fully documented and verifiable test results as well as actual in-field performance data under similar loads and environmental conditions.
Learning to recognize the subtle differences between seemingly identical batteries with thorough due diligence will pay long-term dividends by increasing product longevity to achieve a lower cost of ownership.



Tell Us What You Think!