Battery cells, modules, and systems support many electronic, transportation, and energy applications.
This article briefly reviews the operation of rechargeable batteries and looks at the energy storage value chain; it then presents common battery cell formats and how battery cells are assembled into modules and systems, reviews the development of multi-function structural battery packs, and closes with a look at emerging massless energy storage. Other FAQs in this series dive into materials and fabrication aspects of lithium-ion battery cathodes, anodes, separators, and electrolytes.
The gravimetric density of the energy stored by a battery cell, for example, in mWh/g or Wh/kg, is the product of the cell capacity in mAh/g or Ah/g and the cell voltage. It depends on the electrochemical and physical properties of the active materials in the anode and cathode and the proportion of the active materials to the total cell weight, including the electrolyte, separator, current collectors, packaging, etc. At higher levels of packaging, including battery modules or systems and integrated applications such as electric vehicles, the gravimetric and volumetric energy density decreases with more and more inactive materials and components (Figure 1). As a result, it’s important to have a complete understanding of components such as sensors and cooling devices that are necessary but may or may not be included when comparing the energy storage capabilities of battery modules and systems.
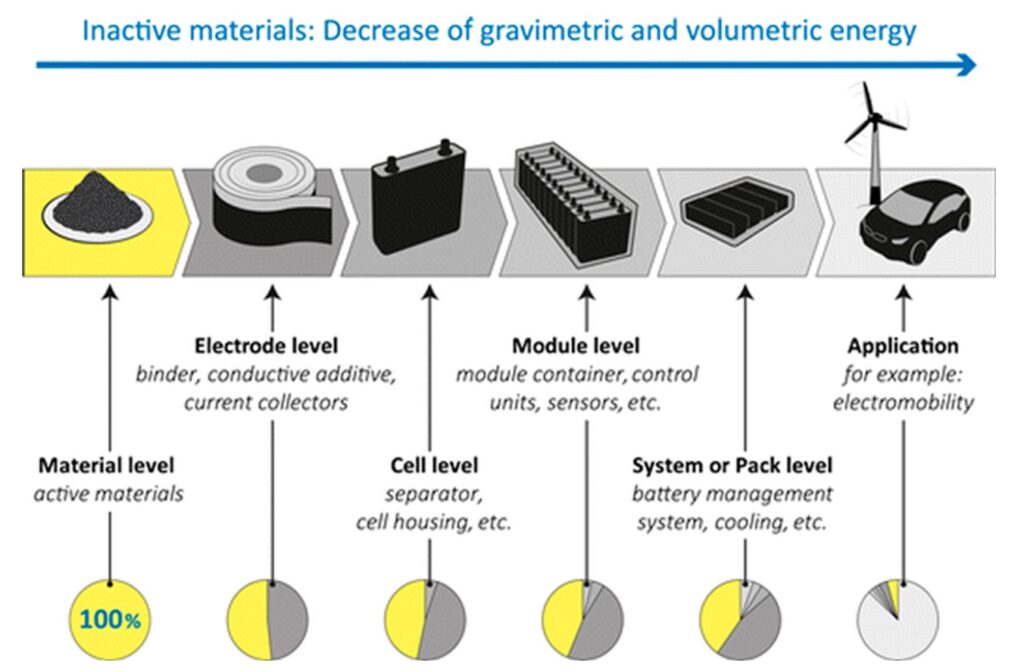
Figure 1: Battery value chain showing the declining percentage of active materials leading to decreasing energy densities moving from the initial materials to the complete system application. (Image: ACS Publications)
The electrochemical capacity (mAh/g) and the open-circuit voltage (VOC) of a cell determine its energy density. The electrochemical capacity is calculated using the number of electrons (or ions, in the case of Li-ion batteries) exchanged per formula weight of the active materials. The VOC is the difference between the electrochemical potentials of the negative and positive electrodes, μN and μP, respectively (Figure 2). VOC should lie within the electrolyte stability voltage window (ESW) for reliable operation. ESW refers to the energy separation between the electrolyte components’ highest and lowest occupied molecular orbitals. During discharge, oxidation and reduction occur at the positive electrode (cathode) and negative electrode (anode), respectively.
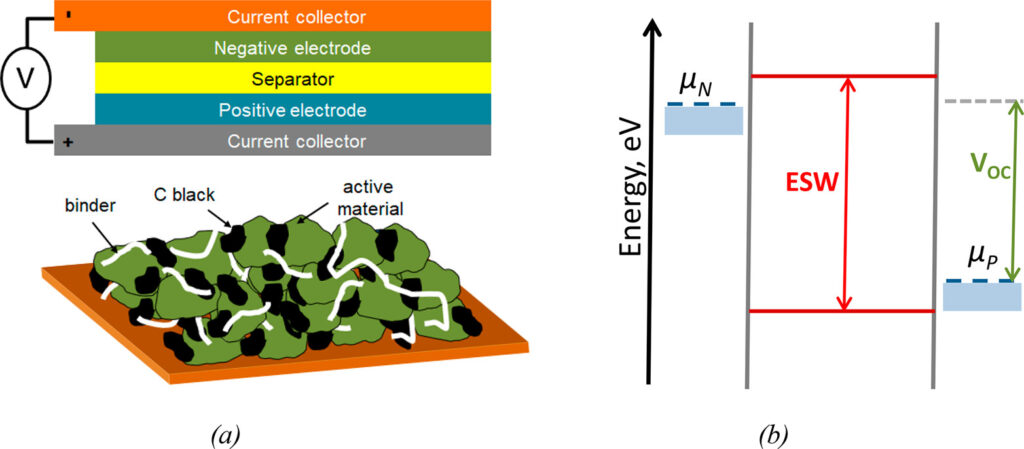
Figure 2: (a) Basic structure of a battery cell (top) and a conventional tape-casted composite electrode on a metal current collector (bottom). (b) Diagram of the electrolyte stability window (ESW) and electrochemical potentials of the negative and positive electrode materials (μN and μP, respectively). (Image: ACS Publications)
Four common packaging technologies for Li-ion cells are coin, cylindrical, prismatic, and pouch. Cylindrical and coin cells are widely used. These packaging formats combine well-established manufacturing technologies with high mechanical stability and can be easily sealed. Cylindrical cells can also include a resealable vent to release pressure under excessive charge. Prismatic cells are newer designs that provide thinner geometries and similar or higher capacities than cylindrical cells. The rectangular format of prismatic cells can facilitate assembly into battery packs.
While coin, cylindrical, and prismatic cells use hard metallic cases, pouch cells use conductive multi-layer foil packages (Figure 3). In a pouch cell, foil tabs are welded to the anode and cathode and sealed to the pouch material to form the contacts. Pouch cells are lighter, lower in cost, and smaller resulting in relatively less inactive materials and higher energy densities.
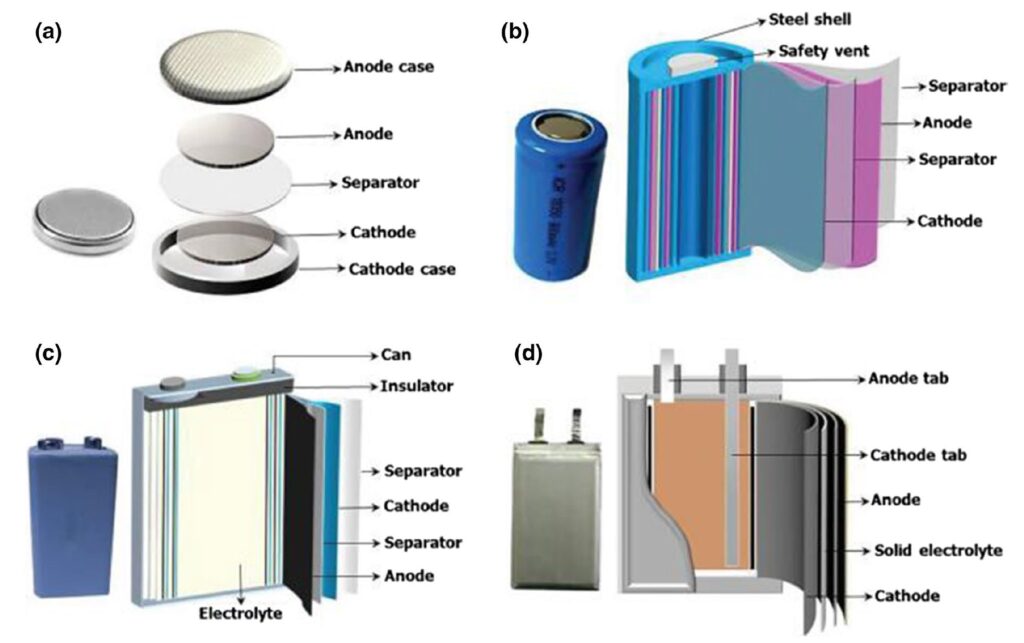
Figure 3: Coin (a), cylindrical (b), prismatic (c), pouch (d) battery package formats. (Image: Springer)
Except when used in small portable devices, individual battery cells need to be packaged into battery packs for integration into the end application device. In addition to multiple cells, a battery pack consists of several elements, including the battery management system (BMS), interconnect system, and housing (Figure 4). Depending upon the application, the BMS can be more or less complex. Basic BMS functions include charge/discharge control, monitoring the state of charge, monitoring the temperature, cell balancing, and providing various safety functions. The connection system connects the cells into a single source of power for the system. The interconnects, called current collectors, can be thin to minimize weight or thicker and heavier to support higher power delivery. In some designs, the current collectors also help move heat away from the battery to keep it cooler. Housings use various materials, from shrink wraps to hard cases, depending on the application’s needs. The housing provides some degree of environmental protection and enables the pack to be handled as a single unit without damaging the BMS or the connection system.
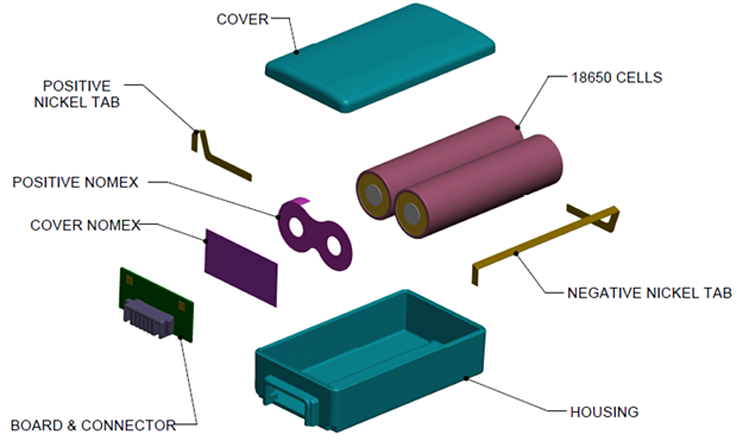
Figure 4: Li-ion battery pack construction using cylindrical 18650 cells. (Image: Inventus Power)
Structural battery packs
Structural battery packs are the next step toward massless energy storage in EVs and electric aircraft applications. Massless energy storage refers to any approach where the battery pack or battery is an integral element in the structural design, effectively reducing the impact of the inactive materials in the energy storage system and contributing little or no incremental weight to the vehicle.
Two approaches have emerged for designing structural battery packs; making the battery pack part of the vehicle structure or making the vehicle structure part of the battery pack. Using an integrated structure such as a honeycomb grid in the battery pack can enable the pack to provide added stiffness and strength to the frame and provide enhanced thermal management for the cells. Or, the battery pack can be sealed to the frame below and the cabin floor above, helping improve thermal management while the cells stiffen the frame and transfer some of the impact in the event of a crash. In either case, these multi-function battery systems provide about 15 percent higher energy densities than traditional EV battery packs.
These lower weight multifunctional battery systems are an important incremental step since a conventional EV battery pack can account for about 25 percent of the total vehicle weight. For EVs, especially for electric aircraft, it will be necessary to increase the total energy storage while reducing the overall vehicle weight. The development of multifunctional materials called ‘structural power composites’ or ‘structural battery composites’ will enable the design of massless energy storage systems.
Massless storage and structural batteries
A common approach to designing structural batteries (SBs) is using carbon fibers in the anode and current collectors to add structural strength. A solid (structural) electrolyte provides ion transport, acts as a separator between the anode and cathode, and provides additional strength to the structure. The cathode may be fabricated on an aluminum (possibly foil) or carbon-fiber substrate. Using these and other structural materials will reduce the number of inactive materials in the energy storage system and support the development of higher performance transportation and other systems (Figure 5). The challenge is to develop structural materials systems with the needed electrochemical and mechanical performance combination that can be delivered at the right cost.
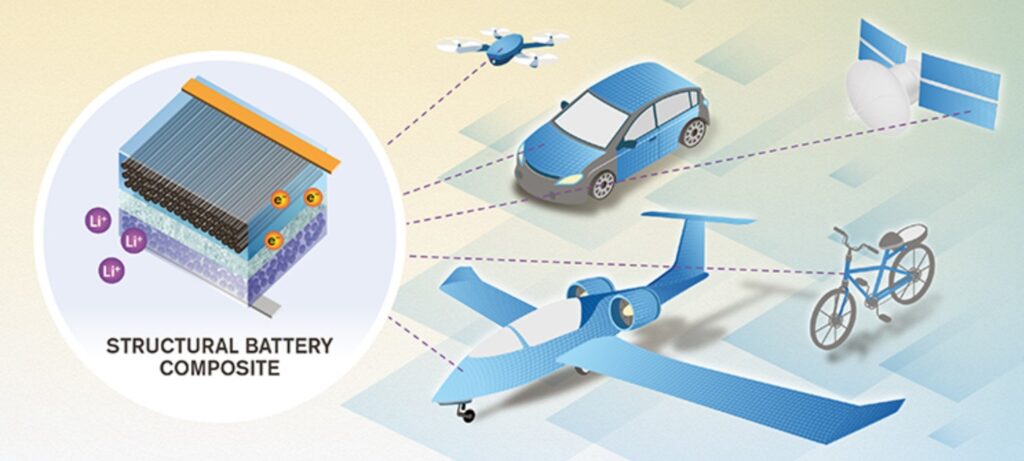
Figure 5: Structural batteries based on carbon fibers will benefit a wide range of applications. (Image Chalmers University)
In one case, a structural battery prototype was developed using a lithium iron phosphate-coated aluminum cathode with an energy density of 24 Wh/kg and stiffness of 25 GPa. That battery has about 20% of the capacity of a comparable Li-ion with a strength comparable with some commonly used materials. In the future, it is projected that the design can deliver an energy density of 75 Wh/kg with a stiffness of 75 GPa, comparable to aluminum.
Another development effort built a Li-metal SB with higher energy density and greater mechanical strength. It delivers a capacity of about 147 mAh/g and has a tensile strength of 168.4 MPa and bending strength of 157.8 MPa. This Li-metal SB includes a carbon fiber woven fabric in the cathode and anode and glass fiber woven fabric in the solid electrolyte and the case. This design combines good load-bearing and energy storage with reliable charge/discharge performance after moderate levels of structural loading, including tension, bending, and compression.
In addition to SBs, there are efforts to develop other types of structural energy storage devices (SESDs) with varying combinations of energy and power densities, including pseudocapacitors and electric double-layer capacitors (Figure 6). Energy densities and structural integrity are important but are only two factors to consider when developing SESDs. A SESD will be expected to have a cycle life comparable to the system’s lifetime in which it’s used; replacement of highly-integrated structural elements could be a costly process. Inherently safe and robust electrochemical systems will be required. Sustainability, recyclability, and lifecycle costs will be important. And it’s likely that the host system’s mechanical architecture will need to be redesigned to gain the maximum benefit from SESDs.
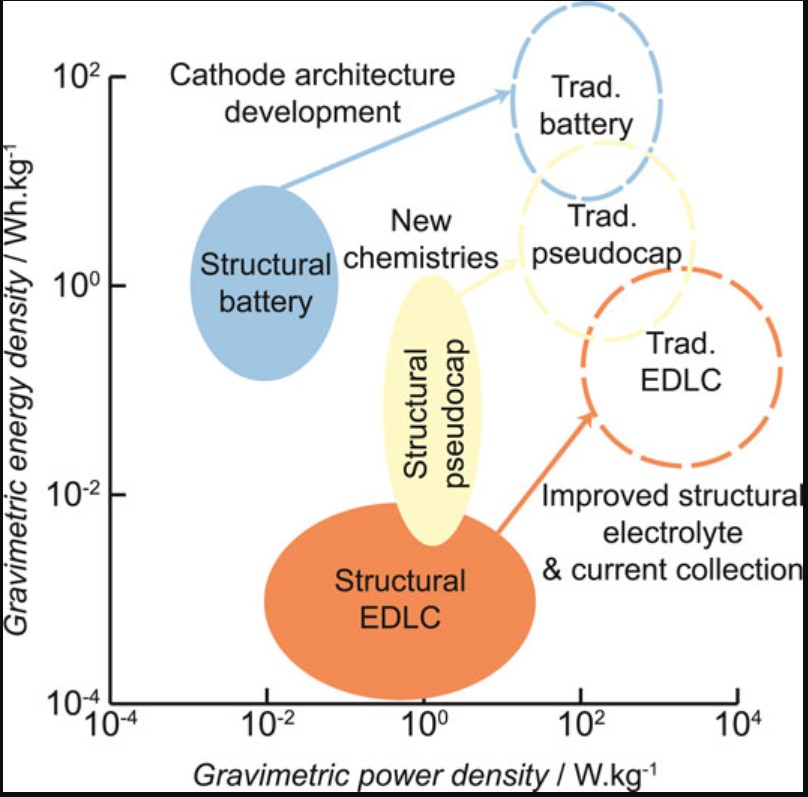
Figure 6: Ragone plot of structural energy storage devices with suggested developments to approach the performance of conventional devices. (Image: Frontiers in Chemistry)
The remaining 4 FAQs in this series review advanced battery materials for cathodes, anodes, separators, and electrolytes. Each FAQ considers how those materials are evolving toward developing solid-state batteries that could form the basis for future massless energy storage systems.
Summary
Li-ion batteries and battery packs continue to evolve. Many current development efforts are focused on reducing the amounts of inactive materials in the completed energy storage system to support increased power and energy densities. In the near-term structural battery packs are being developed to reduce the weight of the batteries in EVs and other transportation applications. In the longer term, SBs and SESDs are expected to improve even greater performance, leading to massless energy storage.
References
A Structural Battery and its Multifunctional Performance, Advanced Energy and Sustainability
Applications of Lithium-Ion Batteries in Grid-Scale Energy Storage Systems, Springer
Battery Materials Design Essentials, ACS Publications
Big breakthrough for’ massless’ energy storage, Chlamers University
Designing Structural Electrochemical Energy Storage Systems: A Perspective on the Role of Device Chemistry, Frontiers in Chemistry
Lithium metal structural battery developed with vacuum bagging, Journal of Materials Chemistry C
The Construction of the Li-ion Battery Pack, Inventus Power




Very helpful
Can you help me I am looking to buy a book I am a process technician