There are numerous cathode materials used in Lithium-ion (Li-ion) batteries optimized for various aspects of performance, but the majority of all Li-ions still use graphite anodes. That may be set to change. The use of graphite with a theoretical gravimetric capacity of about 370mAh/g is being challenged by new materials under development that offer gravimetric capacities 10x higher. This FAQ will review the performance of graphite anodes, look at advanced anode materials and their limitations, with a special focus on Silicon (Si), and close with a brief dive into future possibilities for anode materials, including disordered rock salt.
Graphite anodes are produced by baking graphite onto a copper foil. These anodes are inexpensive, lightweight, porous, durable, and meet the voltage requirements of most Li-ion cathode materials. The graphite anode in a Li-ion battery is composed of layers with sufficient spacing to allow Li ions to insert themselves into the graphic matrix during charging through a process called intercalation (Figure 1). When the battery is discharged, the ions come out of the graphite matrix through a process called deintercalation and flow through the electrolyte and separator to the cathode.
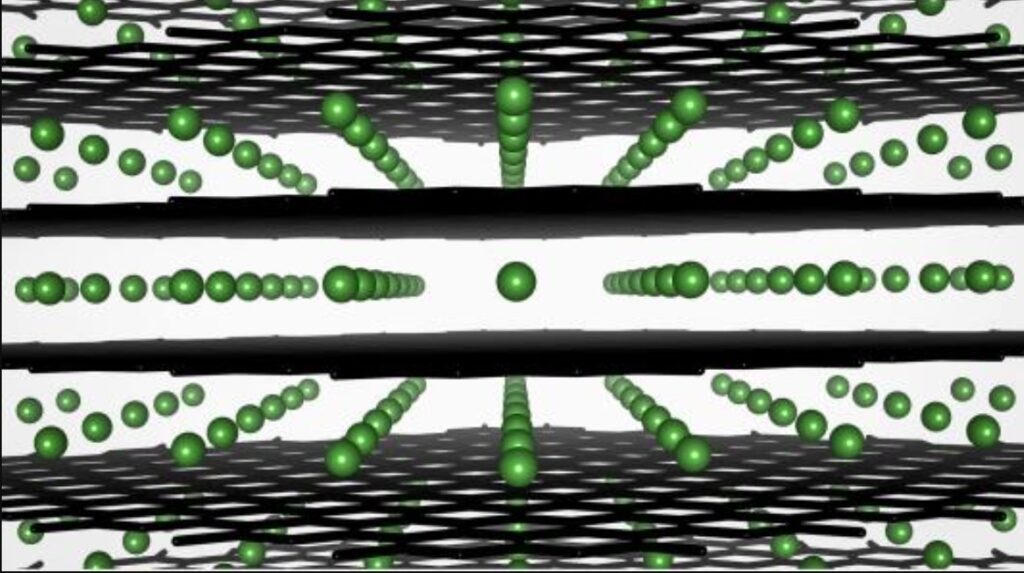
Figure 1: Illustration of the intercalation of lithium ions (green) in a graphite anode. (Image: Argonne National Laboratory)
The processes of intercalation and deintercalation are the keys to Li-ion operation. However, there are limitations. If a Li-ion is charged too quickly, intercalation may not smoothly occur. Instead of penetrating the graphite matrix, the Li-ions can aggregate on the surface of the anode, resulting in an effect called ‘plating’ that can impair or destroy the cell. That places a practical limit on how fast a Li-ion can be charged.
In addition to plating, rapid charging results in a build-up of chemical products from side reactions in the graphite pores, causing irreversible expansion of the graphite and further impairing performance. Beyond a certain point, faster charging causes the graphite to become more atomically disordered and less effective.
Graphite is not perfect, but it works and is cost-effective. Performance characteristics needed by any proposed alternative anode materials include low potential, high capacity, long cycle life, low cost, and strong safety. In addition to graphite, spinel Li4Ti5O12 (LTO) is used in some Li-ion anodes. The tradeoffs inherent between LTO and graphite illustrate the challenge in finding a cost-effective alternative for graphite anodes. LTO is safe, has good cycle life, a more comprehensive operating temperature range, and rate capability better than graphite. As a result, despite its low capacity of only 175mAh/g, compared with 370mAh/g for graphite, LTO finds use in batteries that require fast charging, long lifespans, and high safety. What’s needed is an anode material with all of LTO’s characteristics and higher capacity than graphite.
Silicon is currently the most active area of Li-ion anode development. Si has a high gravimetric capacity, about 4200 mAh/g, the highest capacity compared with other graphite alternatives (Table 1). Si is attracting attention due to its high performance, including high gravimetric capacity, volume capacity of 9,786 mAh/cm3, relatively low working potential (0.5 V vs. Li/Li+), and its natural abundance and low environmental impact. In addition to improving the performance of common Lithium Cobalt Oxide (LCO) cells, Si anodes can increase the appeal of lower energy cathodes such as lithium iron phosphate (LFP), narrowing the performance gap with nickel-manganese-cobalt oxide (NMC) cells, and minimizing the core disadvantage of LFP in applications like electric vehicles. But it’s not that simple.

Table 1: Comparison of Li-ion anode materials options. (Image: MilliporeSigma)
Si is great, but it doesn’t last very long. Very short operating lives for current Si anodes prevent them from being used in commercial Li-ions. There are several contributors to the short operating lives of Si anodes. Si undergoes the highest volume change, 320%, for any alternative anode materials currently under consideration. That high volume change during cycling results in the consumption of Li and electrolyte and causes mechanical stresses in the cell. The combined effect is loss of electrical and ionic conductivity and cell failure. Several possible solutions are being developed, including additives in the electrolyte, increasing the porosity of the Si, or adding conductive and binding materials. Overcoming the negative effects of the volume change is only one aspect of improvising viable Si anodes.
The 300 percent volume changes can literally pulverize the Si anode material, causing poor cycle life. The mechanical fracturing of Si anodes reduces coulombic efficiency and causes an irreversible capacity loss. The solid electrolyte interphase (SEI) breaks as the structure shrinks back during delithiation from the 300 percent volume increase during lithiation. When the SEI breaks, it exposes the Si surface to the electrolyte, and the SEI reforms, causing the SEI to get thicker after each charge/discharge cycle (Figure 2).

Figure 2: Illustration of SEI formation on a pure silicon surface during charge/discharge cycles. (Image: MilliporeSigma)
Li-metal batteries (not Li-ions) using Li-metal anodes also promise higher energy densities. They currently have low cycle lives, and they are prone to dendrite formations which short circuit the cell. Concerns about dendrite formation can be mitigated or eliminated by using solid-state electrolytes (trends in solid-state electrolytes are discussed in “Li-ion Parts 5 – Electrolytes”). Numerous options for solid-state electrolytes are being researched, including sulfides, oxides, phosphates, polyether, polyester, polyurethane, and others. There is no clear leading material; polymers are lower in cost and easier to process than ceramics. But ceramics are more rugged and better suited for challenging environmental conditions and high-temperature operation. The development of Li-metal anodes could result in cells with energy densities of 1,000 to 1,600 Wh/kg and 1,500 to 2,200 Wh/L, 3X to 7X higher than today’s Li-ions.
In addition to high energy density, fast charging is a key performance requirement for future cathode materials. Both Si and Li-metal anodes will likely support some level of fast charging. The open questions are: How fast? And what performance tradeoffs will be needed to support fast charging? Ultimately, one key to the adoption of either Si or Li-metal anodes will depend on how easily they can be integrated into existing battery cell manufacturing processes and produced in large quantities and large cell formats.
Si and Li-metal promise some combination of higher energy density and fast charging, but both currently have limited cycle lives. Applications like electric vehicles require cycle lives beyond 1,000 cycles as well as fast charging. Advanced anode materials using Niobium and Tungsten oxides are under development that are expected to support charging times of about 5 minutes and cycle lives in the tens of thousands. The tradeoff will be energy densities below the 325Wh/L delivered by LiFePO4 (lithium iron phosphate, or LFP) cells that could eliminate these cells from consideration in EV applications.
Future possibilities
Developing carbon-based materials with a larger interlayer distance will help to increase the diffusion rate of Li ions and speed recharging of the cell. One strategy to develop materials with the needed structure is to use nitrogen doping. But controlling the interlayer distance is challenging, and nitrogen doping requires a cleanroom process, increasing costs. Niobium is another material being investigated for high-rate Li-ion anodes. For example, the next generation of Toshiba’s fast-charging SCiB battery is expected to use a niobium titanium oxide anode (NTO) to replace the LTO currently used. NTO can support 3X the storage capacity of LTO.
Nickel niobate (NiNb2O6) is another material with an open and regular crystal structure that can support ultra-fast charging, and its fabrication does not need a cleanroom environment. The minimal volume change enables NiNb2O6 to retain 81% of its capacity after 20,000 cycles at a rate of 100 C. Unfortunately, the capacity of NiNb2O6 is relatively low and varies with the C rate, with a maximum capacity of about 244 mAh/g at 0.5C. Faster C rates such as 1, 5, 10, and 100 C result in lower capacities of 220, 165, 140, and 50 mAh/g, respectively, making it currently unsuitable for commercial use.
The disordered rock salt (DRX) Li3+xV2O5 can be used as a fast-charging anode. For example, a Li-ion with a Li3V2O5 anode yields a cell voltage much higher than a battery using an LTO anode (Figure 3). Li3V2O5 can support over 6,000 charge–discharge cycles with negligible capacity decay and exhibits exceptional rate capability, delivering over 40 percent of its capacity in 20 seconds.
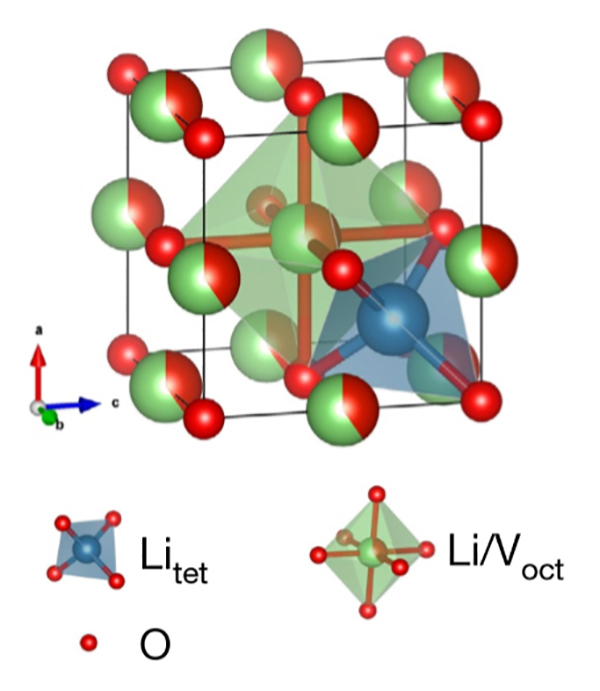
Figure 3: The crystal structure of disordered rocksalt -Li3V2O5. The red balls represent O, the blue tetrahedron represents Li in tetrahedral sites, and the green octahedron represents the Li/V shared octahedral sites. (Image: UC San Diego)
DRX eliminates the concern of lithium metal plating at high charge rates that makes the use of graphite potentially unsafe, and it can store much more energy compared with LTO. The unique redistributive lithium intercalation mechanism of DRX and its low energy barrier is the source of the performance advantages compared with graphite and LTO.
DXRs are also being considered for use in Co-free cathodes that may substantially improve the sustainability and reduce the cost of Li-ions (see “Li-ion Parts 2 – Cathodes”)
Summary
Compared with the long-term and continuous development of cathode materials, anodes have been a relatively quiet aspect of Li-ion technology, with the majority of batteries continuing to use basic graphite anodes. That has changed. Today, anode materials represent a very active area for material development. Si anodes promise near-term advances in Li-ion performance with more exotic materials, including nitrogen doping, the use of various niobium compounds, and DRXs pointing toward very long cycle lives at extremely fast charge and discharge rates.
References
A disordered rock salt anode for fast-charging lithium-ion batteries, Nature
Are Advanced Anode Technologies the Way Forward for Li-ion Batteries?, IDTechEx
Nickel Niobate Anodes for High Rate Lithium-Ion Batteries, Advanced Energy Materials
Recent Developments in Silicon Anode Materials for High Performance Lithium-Ion Batteries, MilliporeSigma
Scientists identify another reason why batteries can’t charge in minutes, Argonne National Laboratory



Tell Us What You Think!