Dendrites are projections of metal that can build up on the surface of lithium anodes and penetrate into the battery electrolyte, eventually crossing from one electrode to the other and shorting out the battery cell. What gives rise to these metal filaments has been somewhat of a mystery, and there has been little progress on how to prevent them.
That situation may change thanks to researchers at the Massachusetts Institute of Technology and Brown University. MIT Professor Yet-Ming Chiang, graduate student Cole Fincher, and five others at MIT and Brown University say they have figured out what causes dendrite formation, at least in solid-state lithium batteries. Writing in the journal Joule, they also claim to show how dendrites can be prevented from crossing through the electrolyte.
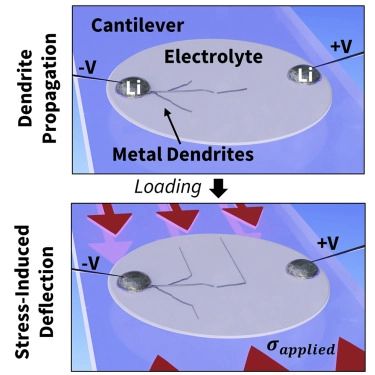 During the process of charging and discharging the battery, ions of lithium move between the anode and cathode. This shuttling back and forth of ions causes the volume of the electrodes to change. That inevitably causes stresses in the solid electrolyte, which must remain fully in contact with both of the electrodes. Chiang says, “There’s an increase in volume on the side of the cell where the lithium is being deposited. And if there are even microscopic flaws present, this will generate a pressure on those flaws that can cause cracking.”
During the process of charging and discharging the battery, ions of lithium move between the anode and cathode. This shuttling back and forth of ions causes the volume of the electrodes to change. That inevitably causes stresses in the solid electrolyte, which must remain fully in contact with both of the electrodes. Chiang says, “There’s an increase in volume on the side of the cell where the lithium is being deposited. And if there are even microscopic flaws present, this will generate a pressure on those flaws that can cause cracking.”
Some researchers had thought that dendrites formed by a purely electrochemical process, rather than by a mechanical one, but the team’s experiments demonstrate that it is mechanical stresses that cause the problem. Those stresses, the team has now shown, cause the cracks that allow dendrites to form. They claim the solution to the problem turns out to be more stress, applied in just the right direction and with the right amount of force.
The process of dendrite formation normally takes place deep within the battery cell and cannot be observed directly, so Fincher developed a way of making thin cells using a transparent electrolyte, allowing researchers to see and record the whole process. “You can see what happens when you put a compression on the system, and you can see whether or not the dendrites behave in a way that’s commensurate with a corrosion process or a fracture process,” he says.
The team demonstrated that they could directly manipulate the growth of dendrites simply by applying and releasing pressure, causing the dendrites to zig and zag in perfect alignment with the direction of the force. Applying mechanical stresses to the solid electrolyte doesn’t eliminate the formation of dendrites, but it does control the direction of their growth. This means they can be directed to remain parallel to the two electrodes and kept from ever crossing to the other side, and thus rendered harmless.
In their tests, the researchers applied pressure by bending the material, which was formed into a beam with a weight at one end. But they say that in practice, there could be many different ways of producing the needed stress. For example, the electrolyte could be made with two layers of material that have different amounts of thermal expansion to produce an inherent bending of the material, as takes place in electromechanical thermostats. Another approach would be to dope the material with atoms that would leave it in a permanently stressed state–the same method is used to produce the super-hard glass for smart phone screens. And the amount of pressure needed is not extreme: The experiments showed that pressures of 150 to 200 MPa were sufficient to stop the dendrites from crossing the electrolyte. That’s “commensurate with stresses that are commonly induced in commercial film growth processes and many other manufacturing processes,” so should not be difficult to implement in practice, Fincher adds.
In fact, a different kind of stress, called stack pressure, is often applied to battery cells, by essentially squishing the material in the direction perpendicular to the battery’s plates — somewhat like compressing a sandwich by putting a weight on top of it. It was thought that this might help prevent the layers from separating. But the experiments have now demonstrated that pressure in that direction actually exacerbates dendrite formation. “We showed that this type of stack pressure actually accelerates dendrite-induced failure,” Fincher says.
Instead, pressure must be in the direction of the plane of the plates, like a sandwich squeezed from the sides. That could finally make it practical to produce batteries using solid electrolyte and metallic lithium electrodes.
The next step will be to apply these principles to the creation of a functional prototype battery, Chiang says, and then to figure out exactly what manufacturing processes would be needed to produce such batteries in quantity. Though they have filed for a patent, the researchers don’t plan to commercialize the system themselves. “I would say this is an understanding of failure modes in solid-state batteries that we believe the industry needs to be aware of and try to use in designing better products,” he says.
The research team included Christos Athanasiou and Brian Sheldon at Brown University, and Colin Gilgenbach, Michael Wang, and W. Craig Carter at MIT. The work was supported by the U.S. National Science Foundation, the U.S. Department of Defense, the U.S. Defense Advanced Research Projects Agency, and the U.S. Department of Energy.



Tell Us What You Think!