When discussing batteries, a lot of technical terms are used. If you’re not familiar with these terms, it can be challenging to understand a discussion of battery technology. This page lists some of the most important battery-related terms:
- Conventional Current: Electricity is conventionally considered to flow from positive to negative. However, because electrons are negatively charged, the actual flow of the charge carrying electrons is in the opposite direction – electrons flow from negative to positive.
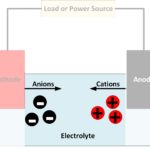
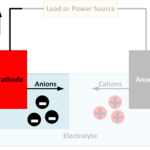
- Ions are electrically charged atoms or molecules. They are charged because they have either gained electrons (negatively charged) or lost electrons (positively charged).
- Anions are negatively charged ions, which have gained electrons.
- Cations are positively charged ions, which have lost electrons.
- Redox is short for a reduction-oxidation, a type of chemical reaction in which ions are formed.
- Oxidation is a redox reaction in which electrons are lost, creating cations. Don’t be confused by the name; this does not have to involve oxygen. The resulting chemical is said to be oxidized.
- Reduction is a redox reaction in which electrons are gained, creating anions. The resulting chemical is said to be reduced.
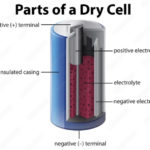
- Electrolytes are substances that separate into cations and anions, enabling current to flow through the movement of these ions in opposite directions. Typically electrolytes are liquids in rechargeable batteries.
- Electrodes are where the electrical circuit makes contact with the electrolyte – the chemicals used to store chemical energy.
- Anodes are the electrodes where the oxidation reaction occurs, producing anions. Conventional current enters the battery at the anode while electrons leave the battery. The negative battery terminal is at the anode, but internally, the anode can be considered positive since electrons enter the electrolyte. When recharging a battery, the current reverses, and the anode, therefore, becomes a cathode. However, it may still be referred to as the anode.
- Cathodes are electrodes where the reduction reaction occurs. Conventional current leaves the battery at the cathode while electrons enter the battery. The positive battery terminal is at the cathode, but internally, the cathode is positive since electrons leave the electrolyte.
- Intercalation is a reversible process where ions are inserted into a layered material. This is the process by which ions are often stored at the electrodes of a battery.

Tell Us What You Think!