There are many alternatives to Li-ion batteries, including fuel cells, various types of supercapacitors, redox flow batteries, novel Li-based chemistries such as lithium-sulfur (LiS), and more. This FAQ focuses on alternative non-lithium rechargeable battery chemistries, including calcium-ion (Ca-ion), magnesium-ion (Mg-ion), sodium-ion (Na-ion), zinc-ion (Zn-ion), iron-air (Fe-air), and sodium-sulfur (NaS) that can be more readily integrated into existing battery recharging technologies and infrastructures, while avoiding the cost and safety challenges associated with most Li-based battery chemistries.
Two of the future’s largest uses for rechargeable batteries will be electric vehicles and grid-scale energy storage to support renewable energy generation. While not every alternative chemistry is suitable for EVs, their use in energy storage systems will help relieve pressure on the available lithium supply and help maintain lower overall battery pricing (Figure 1).
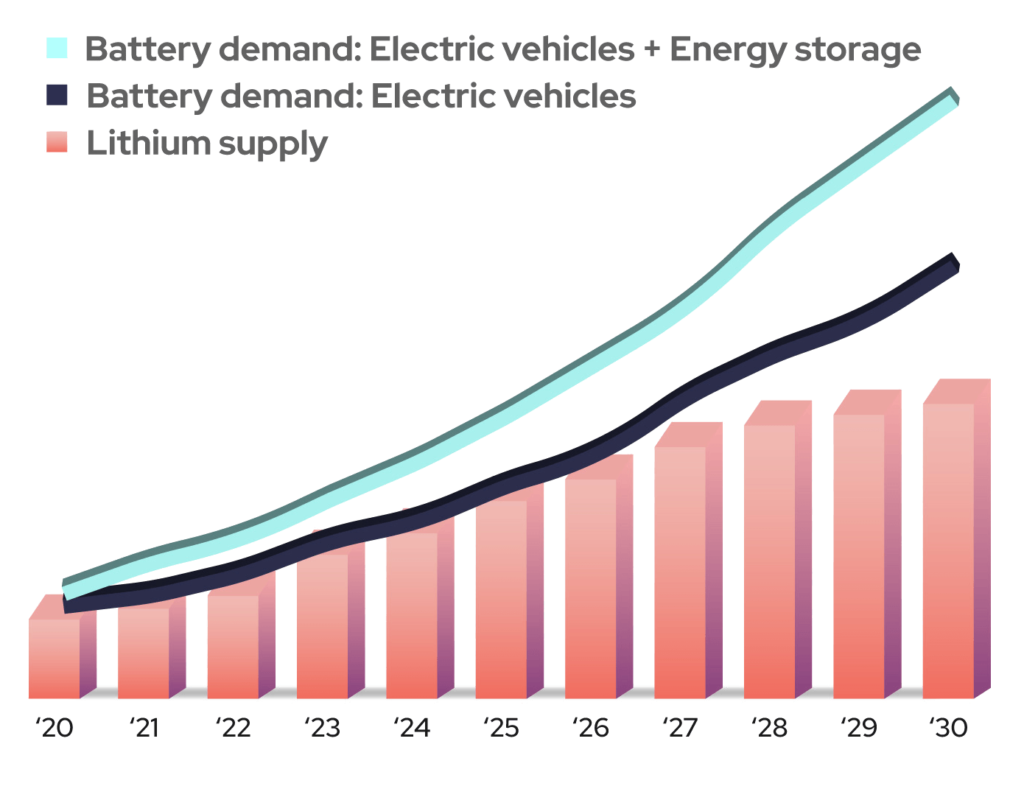
Figure 1: Demand for Li-based batteries is projected to be significantly higher than the available lithium supply can support for the foreseeable future. (Image: Salient Energy)
First-order figures of merit (FOM) for comparing rechargeable batteries include the reduction potential, which determines the cell voltage and the theoretical anode capacity. For example, Li and Ca are comparable in terms of reduction potential, but Li has a much larger gravimetric capacity (Figure 2). Li has a theoretical gravimetric capacity of 3861 mAh/g; Al is next with 2980 mAh/g, followed by Mg with 2205 mAh/g. The theoretical volumetric capacities of Al, Zn, and Mg are 8046, 5851, and 3833 mAh/L, respectively. At first glance, Ca K and Na do not look promising.
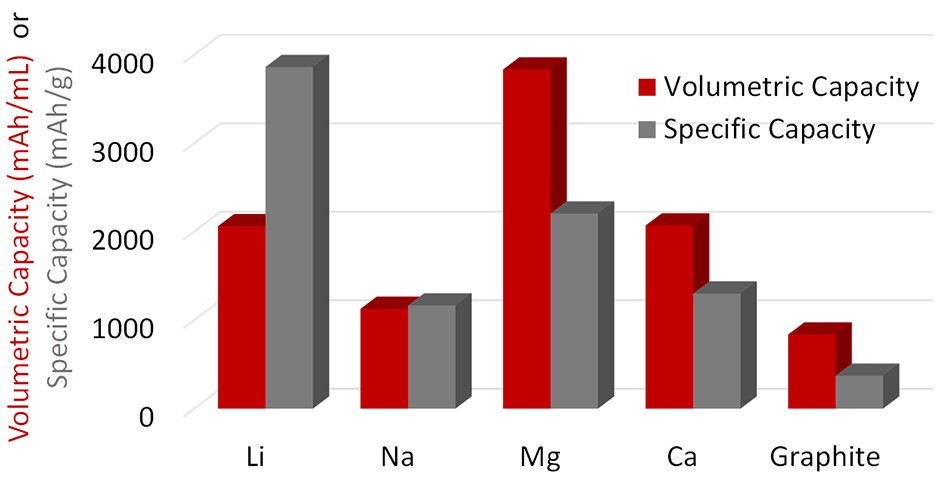
Figure 2: Theoretical capacities for selected rechargeable battery systems. (Image: Advances in Mechanical Engineering)
The theoretical FOM values may not provide an accurate picture of the performance of actual batteries. For example, the usable content for a metal anode is far less than 100 percent and differs between the various metals. Real discharge capacities are significantly lower and often nullify the theoretical differences. And regardless of the capacity of the anode, battery performance is also strongly impacted by the charge/discharge capacity of the cathode, specifically related to the intercalation processes of the cathodes, which varies from metal to metal. Finally, irrespective of the theoretical performance, some chemistries (and therefore some batteries) are much less expensive and environmentally impactful than others.
Ca-ion & Mg-ion are future possibilities
Ca and Mg are promising metals for future rechargeable batteries. Ca is the most abundant alkaline element and fifth most abundant metal in the Earth’s crust, while Mg is the eighth-most abundant element. Replacing Li with either Ca or Mg would eliminate the supply risk from depletion and result in lower-cost batteries.
Ca battery energy densities have been demonstrated up to 250 Wh/kg, with discharge charge capacities up to 250 mAh/g and operating voltages up to 4 V. Current densities up to 500 mA/g have been demonstrated, and discharge rates over 5C have been realized with slightly lower capacity compared with Li batteries.
Mg is divalent; its oxidation results in two electrons and an Mg2+ ion. As a result, Mg has a theoretical volumetric capacity compared with Li. Even though the theoretical capacities may not be achievable, a higher theoretical value promises higher practical performance. On the other hand, Mg is heavier than Li, so that an Mg battery would be heavier for a given energy capacity level. Today’s Li-ion batteries deliver less than 150 mAh/g, while a theoretical Mg-ion battery with a sulfur cathode is projected to deliver 1000 mAh/g. Replacing Li-ion with Mg-ion in an electric vehicle might significantly increase the driving range.
In addition to their other benefits, Ca and Mg batteries are expected to be safer than Li-ions and have longer operating lives than Li-ions. It’s also expected that Mg-ion will be much faster charging than Li-ion.
Prussian blue, Prussian white, and Na-ions
Na-ion batteries are a relatively new chemistry for commercial cells and could be a competitor to Li-ions in EVs, power tools, and other applications. Prussian blue (PB) is a blue paint pigment that is being adapted for use in rechargeable batteries. PB and its analogs are being used to develop Na-ion batteries with high working potentials, high theoretical capacities, and low toxicity. One of the analogs, Prussian white (PW), is a fully reduced and sodiated form of PB and is currently being used in commercial rechargeable batteries. Challenges with fabricating batteries using PW include the need for a high-temperature, high-pressure, and oxygen-free environment.
Current Na-ion batteries have higher costs and lower energy densities with similar power delivery capabilities compared with Li-ions. Na is a highly available element and the cost of Na-ion batteries is expected to decline in the future, making Na-ion batteries competitive with Li-ions.
Performance advantages claimed for Na-ion include fast charging, improved thermal stability, lower temperature operation, and ease of integration. The energy density of current Na-ions is up to 160Wh/kg, and at 25 °C, the battery can charge to 80% in 15 minutes.

Figure 3: Comparison of Na-ion with Li and Pb rechargeable batteries. (Image: Wood Mackenzie)
Zn-ion batteries
Zi-ion is another chemistry that has recently emerged as a possible contender to replace Li-ions. The materials used to fabricate Zi-ion batteries are abundant and could lead to lower cost energy storage. In addition, water-based Zi-ion batteries can be fabricated using equipment very similar to that used to make Li-ions, but Zi-ions are simpler and safer to fabricate. The use of existing production equipment will enable Zi-ions to scale-up production rapidly. In addition, Zi-ions do not require formation cycling and can move more quickly and with less cost from the end of the production line into applications. Current Zn-ion production lines are claimed to produce two-thirds less greenhouse gas emissions compared with Li-ion production.
Zn-ion cells have been fabricated with an energy density of about 450 Wh/L and capacity retention of more than 80 percent over 1,000 cycles, with no dendrite formation at the Zn electrode. Zn-ions use a water-based electrolyte and are intrinsically safe. They can be used in areas where Li-ion adoption has been limited as a result of safety concerns. Combined with the low cost of the materials used to fabricate Zn-ion batteries, their long service lives are expected to make them a cost-effective choice in grid-scale energy storage applications.
Reversible rusting
Iron-air batteries produce electricity through the oxidization (rusting) of Fe. The oxygen comes from the ambient atmosphere, eliminating the requirement for the cell to store it and resulting in energy densities up to 1,200 Wh/kg — twice the energy density of typical Li-ions. Iron-air batteries are expected to operate for about 30 years.
Existing iron-air batteries can supply 100 hours of energy at a cost that is claimed to be comparable to conventional power stations and less than one-tenth the cost of Li-ions. Iron-air batteries are also rugged and resist damage from overcharging and over-current conditions.
Using a non-flammable, water-based electrolyte, each iron-air cell is about one cubic meter in size. An iron-air battery consists of a stack of 10 to 20 cells. Iron air batteries are assembled in modular MW-scale systems, with a typical installation consisting of hundreds of batteries. Depending on the configuration, iron-air batteries can store from 1 to 3 MW per acre.
High operating temperature alternative
NaS batteries are another alternative to Li-ion. Compared with Li, Na and S are abundant and inexpensive, reducing supply chain concerns. The largest grid-scale battery energy storage system (BESS) in operation uses NaS batteries and is 5-times larger than the second-largest BESS that’s based on Li-ions. NaS batteries have Na as the negative electrode and S for the positive electrode. Alumina ceramic is used as the electrolyte, allowing only Na ions to pass through (Figure 3). When the battery is discharged, the Na oxidizes and the S is reduced to polysulfide (Na2Sx). The charging step recovers metallic Na and elemental S. The battery operates at 300 °C, and both elements are liquid when the battery is operating (Figure 4). NaS batteries have operating lives of 15 years, compared with less than 3 years for Li-ions.
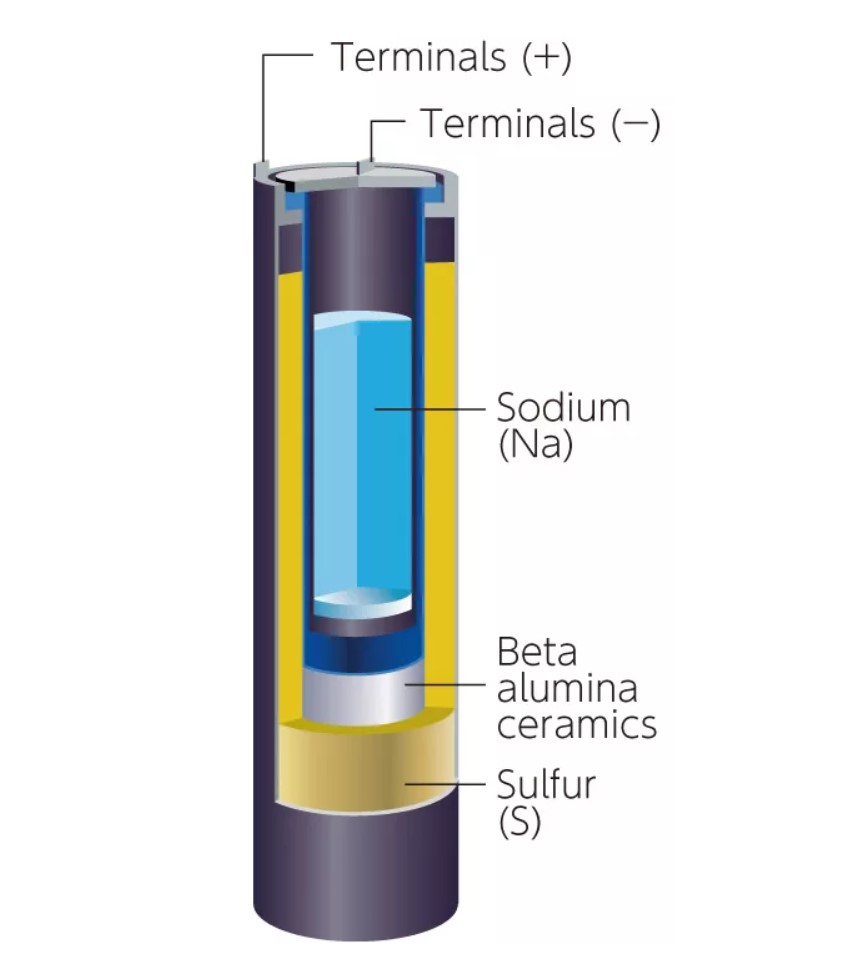
Figure 4: NaS batteries use an alumina tube as the electrolyte and operate at 300 °C. (Image: BASF)
NaS-based BESS are packaged in containers that can deliver 250 kW with a capacity of 1.45 MWh. The containers have been tested for self-extinguishing capabilities and mechanical stability and the NaS battery cells are certified to UL1973.
Summary
Li-ion batteries have been, and continue to be, a highly successful energy storage technology. However, the environmental impact of producing and using Li-ions combined with growing cost and supply chain concerns are resulting in the emergence of a growing number of contenders to replace Li-ions. This FAQ has presented chemistries in early commercial production as well as those still under development. In each case, the new chemistries promise to address the supply chain issues associated with Li-ions. Li-ion will remain the dominant rechargeable battery chemistry for now. But in the future, designers will have a growing number of alternatives, and cost/performance tradeoffs, to consider.
References
Containerized NAS Batteries, BASF
Iron-air battery, enabling a 100% renewable grid, Form Energy
Magnesium-ion batteries for electric vehicles: Current trends and future perspectives, Advances in Mechanical Engineering
Prussian Blue Analogs as Battery Materials, Joule
Prussian White with Near-Maximum Specific Capacity in Sodium-Ion Batteries, ACS Publications
Sodium-Ion Batteries Poised to Pick Off Large-Scale Lithium-Ion Applications, IEEE Spectrum
The Promise of Calcium Batteries: Open Perspectives and Fair Comparisons, ACS Energy Letters
Will sodium-ion battery cells be a game-changer for electric vehicle and energy storage markets?, Wood Mackenzie
Zinc-ion battery, Salient Energy




Tell Us What You Think!