Designers have choices and tradeoffs when choosing the ideal Li-ion battery chemistry. Batteries affect the cost, lifetime, and usefulness of an application. An optimal energy storage system is critical to ensure the life and performance of an application, so it’s essential to start with the best battery chemistry for the job.
This FAQ reviews six performance aspects of Li-ions that designers should consider when choosing a Li-ion battery chemistry. Typically, Li-ions are preferred over alternative rechargeable batteries because they’re lightweight and can store large quantities of energy. Both of these characteristics are desirable in most applications. However, there are different Li-ion chemistries, which offer varying levels of performance and cost depending on the materials used in the cathode, the anode, and the electrolyte.
The six considerations when analyzing the suitability of Li-ion chemistries include (Figure 1):
- Nominal voltage
- Capacity, including specific energy, the energy per unit mass measured in Wh/kg, and specific power, the power per unit mass measured in kW/kg
- Lifespan
- Charging and discharging rates
- Cost including acquisition and maintenance costs
- Safety and environmental impact
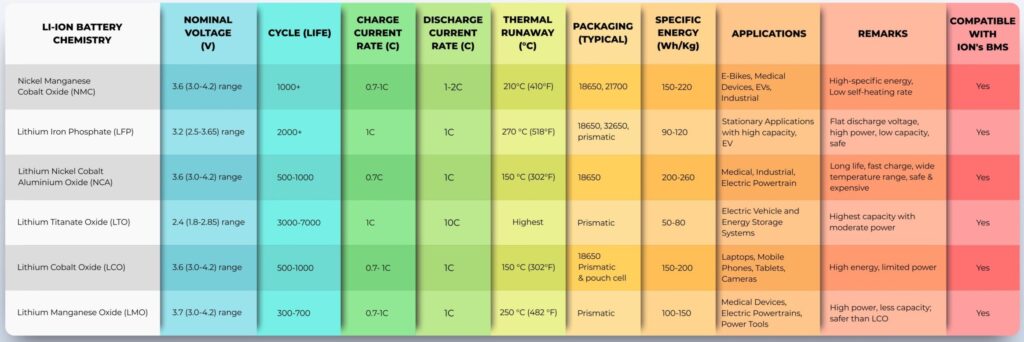
Figure 1: Li-ion batteries are available using a variety of chemistries that deliver a wide range of
performance options. (Image: Maxell Energy Systems)
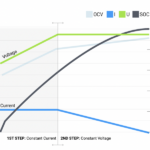
Of course, it’s not quite this simple. Several of these specifications are interrelated. Discharge rates are critical. Charging and discharging rates, measured in mAh or Ah, are measured relative to a cell’s capacity. For example, a cell with a 5.0 Ah capacity can deliver 5.0 A for an hour at 25° C. Its ‘C’ rate for charging and discharging is 5.0 A.
The discharge rate impacts a battery’s voltage and capacity. In addition to different nominal voltages, it can decline at varying rates for different chemistries as a battery is discharged. And the capacity of a Li-ion battery can be strongly impacted by the discharge rate.
Applications that require charging or discharging faster or slower than the nominal C rating can experience different capacities for the same battery. For some chemistries, charging and discharging at high C rates significantly reduces the long-term capacity and lifetime of the battery. Conversely, charging and discharging at low C rates, for example, C/10, can dramatically increase a battery’s lifetime.
The expected operating temperature is another factor to consider. At higher temperatures, users should be concerned with overheating and safety issues. At lower temperatures, the C rate supported by a battery can be reduced.
Over time, all Li-ions lose capacity due to changes in the chemical and physical properties of the anode, cathode, and electrolyte. Managing the depth of discharge (DoD) is critical to maximizing the lifetime of Li-ion batteries. Optimizing the charging scheme, particularly the maximum charging voltage, can significantly enhance the number of cycles reached.
Starting with the ideal battery for a given application is only the first step. Next, it’s important to optimize the battery-management system to ensure the expected performance.
Specific energy and power
Specific energy and specific power reflect a combination of the nominal voltage and the capacity of a battery, usually at a 1C rate. Electrode materials and the designs of Li-ions are optimized over a broader range of specific energy and power levels compared to other battery chemistries (Figure 2).
In addition, Li-ions can be optimized for higher operating temperatures and other desirable characteristics, but often at the expense of other parameters such as C rates. The performance tradeoffs of several common Li-ion chemistries are reviewed below.
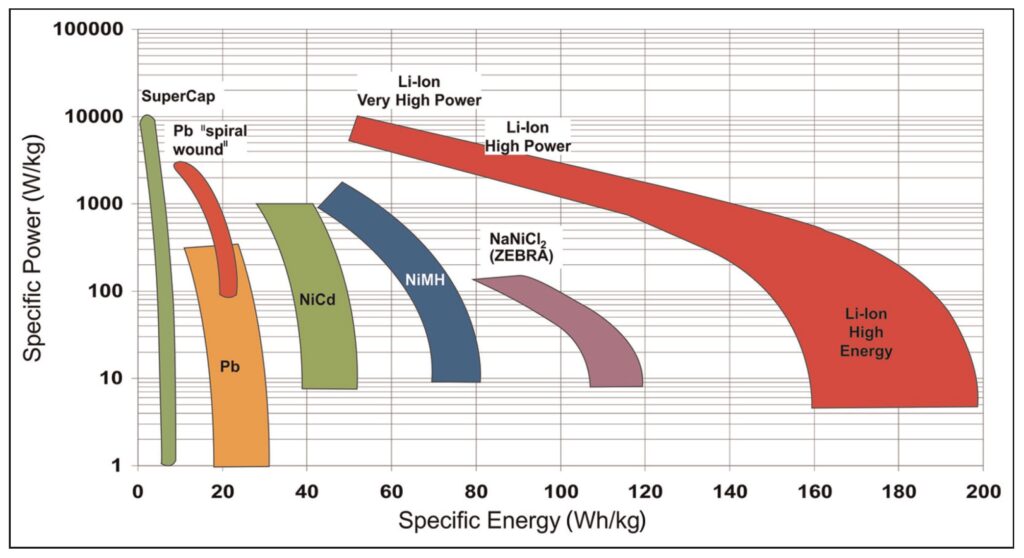
Figure 2: Ragone plot comparison for several rechargeable battery chemistries showing the wide range of performance offered by various Li-ion chemistries. (Image: Journal of Automotive Engineering)
Lithium Cobalt Oxide (LiCoO2) — LCO
LCO batteries have high specific energy but low specific power. This means they do not perform well in high-load applications but can deliver power over a long period. It also means that discharging or charging at high C rates will significantly reduce battery life.
LCO batteries are used in small portable electronics that require no high C rates, benefitting from high specific energy — such as mobile phones, tablets, and laptops. LCOs are losing market share to other Li-ion chemistries because of their relatively short lives (typically under 1,000 cycles), the high cost of the cobalt needed in this chemistry, and the challenges associated with low thermal stability and safety concerns.
Lithium Manganese Oxide (LiMn2O4) — LMO
The manganese oxide forms a 3D-spinel structure that improves ion flow, reducing internal impedance and supporting higher charge and discharge rates. The spinel structure contributes to higher thermal stability compared to LCO and LMO, which can be used at higher temperatures, simplifying battery and thermal management.
Generally, LMO batteries have a lower cycle life. But not all LMO batteries are created equal. LMO chemistry is optimized for high-load applications or longer-life applications.
Lithium Nickel Manganese Cobalt Oxide (LiNiMnCoO2) — NMC
Like LMO batteries, NMC chemistry is optimized for high-energy or high-power applications. NMC batteries combine the benefits of the Ni and Mn in the cathode. Mn provides high stability with a low specific energy. Ni is not as stable but offers higher specific energy. Combined, Ni and Mn offer a more stable battery with a high specific energy.
One disadvantage of MNC batteries is their slightly lower voltage. So, electrolyte optimization is a critical factor in boosting the capacity of these batteries. Overall, MNC batteries provide thermal stability, low self-heating, good cycle life, and the ability to optimize the tradeoff between high energy and power, making them a good choice for industrial applications and EV powertrains.
Lithium Nickel Cobalt Aluminum Oxide (LiNiCoAlO2) — NCA
NCA combines high specific energy, good specific power, and a long life span. This chemistry delivers a relatively high current for extended periods, with a nominal voltage of 3.6V, supporting long-duration power delivery.
Doping the lithium nickel cobalt oxide with aluminum improves performance by stabilizing its thermal and charge transfer resistances. In addition to the basic chemical structure, there are variations in the relative amounts of Ni, Co, and Al in these batteries. Increasing the amount of Ni boosts the output voltage, resulting in a greater quantity of energy stored in the battery. However, higher levels of Ni can reduce battery life and increase sensitivity to thermal breakdown. The Al helps to improve safety and stability but reduces capacity. NCA is gaining use in EVs, and is the chemistry used by Tesla.
Lithium Iron Phosphate (LiFePO4) — LFP
The nano-scale Li-phosphate cathodes in LFP combine low impedance with decent electrochemical performance, which results in a high current rating, long lifecycle, good thermal stability, enhanced safety, and tolerance in case of abuse. But these batteries only offer a moderate level of specific energy and are sensitive to moisture, which reduces cycle life. Their performance also declines at low temperatures.
LFP batteries have flat discharge curves and lives of 2,000 cycles or more (if protected from moisture). In certain applications, LFPs replace lead-acid batteries. Compared with lead-acid batteries, the lifetime of LFP batteries is insensitive to high levels of DoD. Most of these batteries are rated for operation at 80% DoD, and some designs allow 100% DoD without damaging the battery.
LFPs are one of the safest Li-ions on the market. While they are often used to replace lead-acid batteries, their low specific energy and poor performance at low temperatures mean LFP batteries are not suited for high-cranking applications.
Lithium Titanate (Li2TiO3) — LTO
LTO batteries are available from several companies and differ from the already discussed chemistries. An LTO battery can have an LMO or NMC cathode. But the typical graphite anode is replaced with Li-titanate with a spinel structure.
LTOs can be fast-charged, discharging at 10 C or faster for a higher power density. These batteries have a lower energy density due to their 2.4V nominal voltage. LTO cells can last up to 7,000 charge cycles at 25° C. When charged and discharged at 55° C, the cells still offer a life cycle of about 1,000 cycles before reaching 80% capacity. They also have superior, low-temperature rate capability and are among the safest Li-ions. In exchange, they tend to be costly.
LTO batteries are used in several applications, including EV powertrains, uninterruptible power systems, renewable energy storage, telecommunications installations, aerospace, satellite, and military systems.
Summary
Li-ion batteries are available in different chemistries that deliver various performance levels (Figure 3). Six considerations when selecting specific Li-ion chemistry include: nominal voltage, capacity including specific energy and specific power, lifespan or cycle life, charging and discharging rates, as well as cost and safety, and environmental impacts. The tradeoffs between features are not fixed. Fortunately, ongoing technological developments and new chemistries will lead to new options for designers.
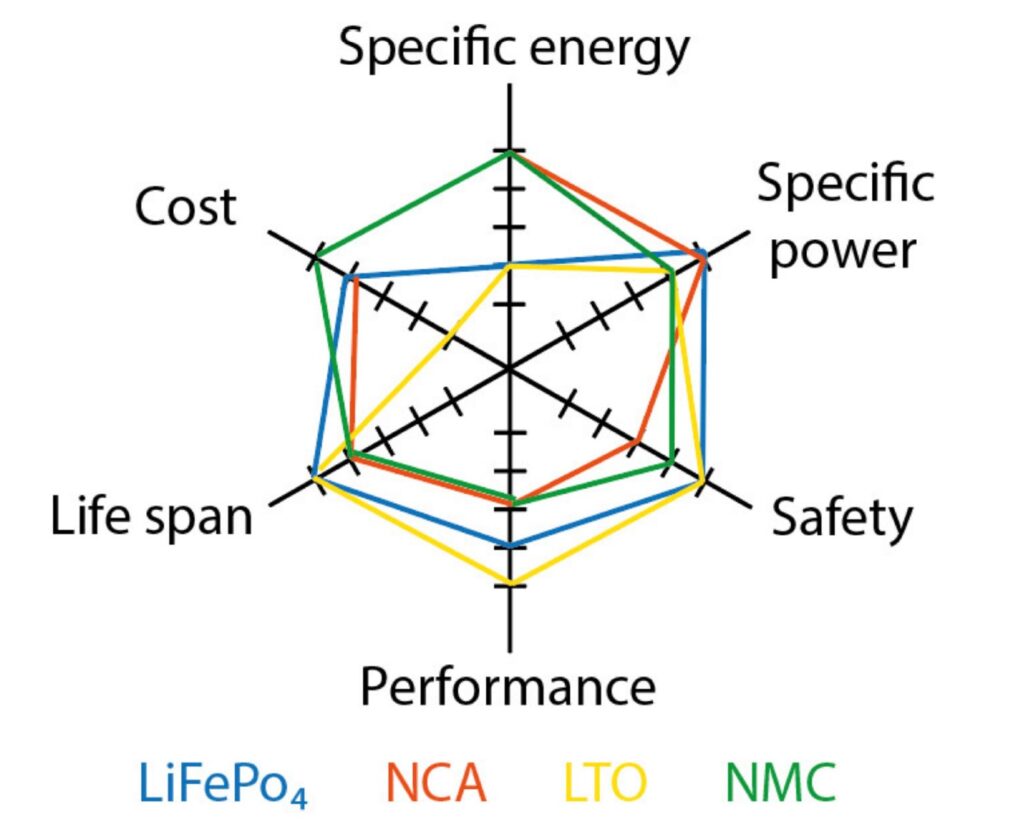
Figure 3: A comparison of the performance characteristics of certain Li-battery chemistries. (Image: MDPI energies)
References
- A review of current automotive battery technology and future prospects, Journal of Automotive Engineering
- Current Li-ion battery technologies in electric vehicles and opportunities for advancements, MDPI energies
- Design and implementation of an electric skibus line in north Italy, MDPI energies
- Selecting the Right Lithium-Ion Battery for Your Application, Maxwell Energy Systems


Tell Us What You Think!