A battery is not an ideal finite power supply. The energy stored in a fully charged battery cannot be supplied to the digital circuitry to its full extent because the amount of energy a battery can provide depends on the current drawn from the battery itself. In other words, the higher the discharge current, the higher the energy waste of the battery.
For example, alkaline batteries generally have a service life of seven to 10 years. Lithium BR and CR batteries can last about 10 to 15 years, and lithium-thionyl-chloride cells can last more than 20 years.
General terms
Before we go any further, we should know the following terms.
- Nominal Voltage: Voltage of a fully charged cell across the positive and negative terminals of the battery.
- Energy/Battery Capacity: The energy stored in a battery is called the battery capacity.
- Energy Density: The energy density is the measure of how much energy a battery contains in proportion to its weight. Higher the energy density of the battery, the more costly the battery technology is.
- Self-Discharge Rate: Batteries do not last forever. Even if they remain unused, electrochemical reactions are still taking place, slowly draining the battery naturally. This process is called the self-discharge rate.
- Shelf Life: Battery shelf life is the length of time a battery can remain in storage without losing its capacity.
- Battery Life: It is the run time on a full charge battery in mAh.
Battery life calculation
The battery life can be calculated using the battery’s input current rating and the load current of the circuit. Battery life is inversely proportional to load current which means, it will be high when the load current is low and it will be low if the load current is high.
The capacity of the battery can be measured from the following formula:
Battery Life = Battery Capacity( mAh )/ Load Current (mA) * 0.9
*The factor of 0.9 makes allowances ( temperatures, aging, etc.) for external factors which can affect battery life.
For example, 2500mAh(2.5Ah) battery with a device that draws 500mA(0.5A) you have:
2.5Ah/0.5A * 0.9 = 4.5 hours
Classifications of batteries
There are two classifications of batteries:
Primary battery. Primary batteries are commonly known as dry cells. A primary battery is a convenient source of power for portable electronics and devices. They are the type to “discard immediately when discharged”. These batteries are basically the source of dc power. Key features of primary batteries are inexpensive, lightweight, convenient to use, and require no maintenance.
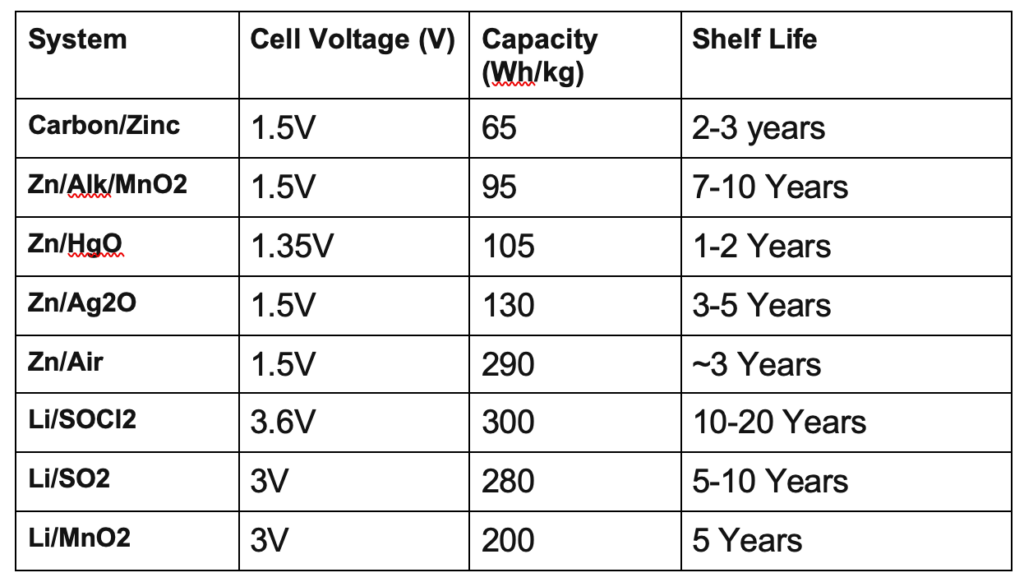
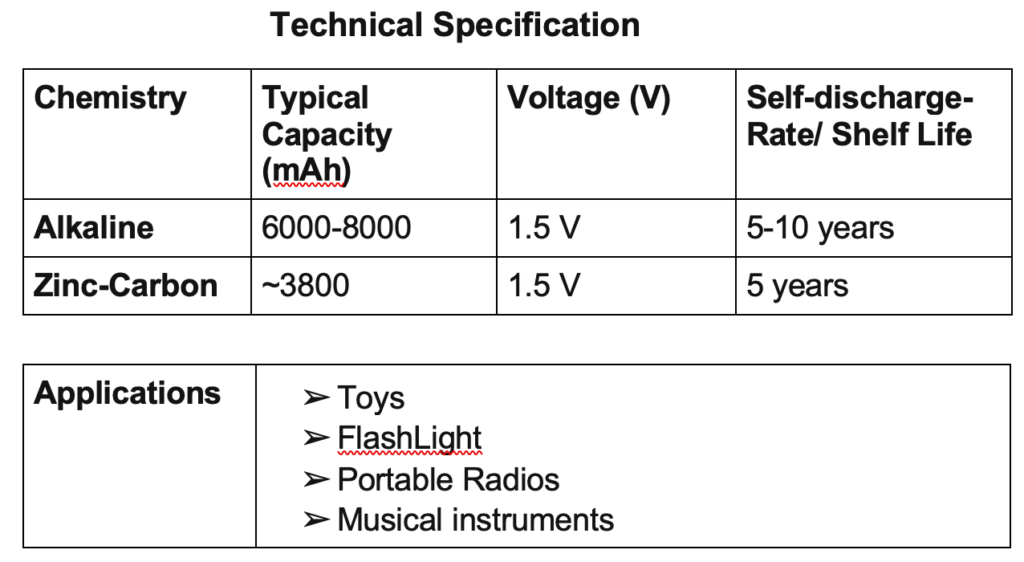
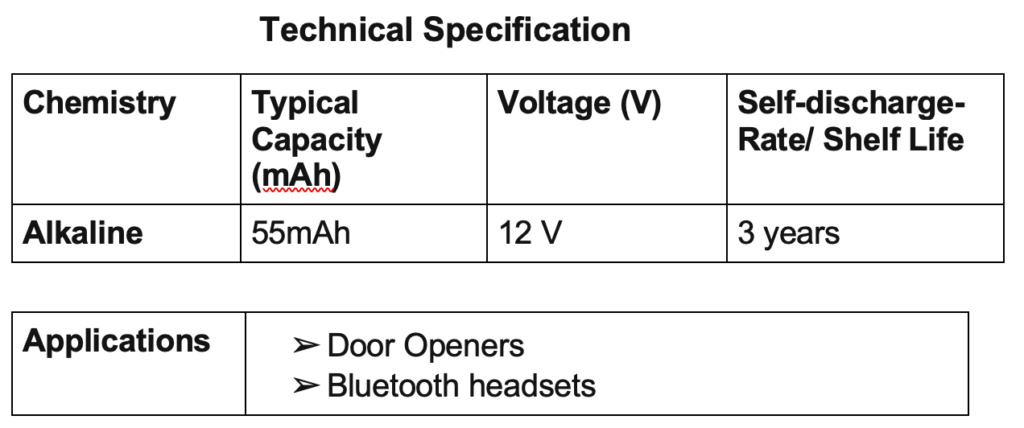
Examples are zinc-carbon cells, metal–air-depolarized batteries, and alkaline zinc–manganese dioxide cells. The most common primary batteries are the 1.5 V alkaline batteries (AA, AAA, AAAA, C, D, 9V).
On the basis of chemical compositions, types of primary batteries are:
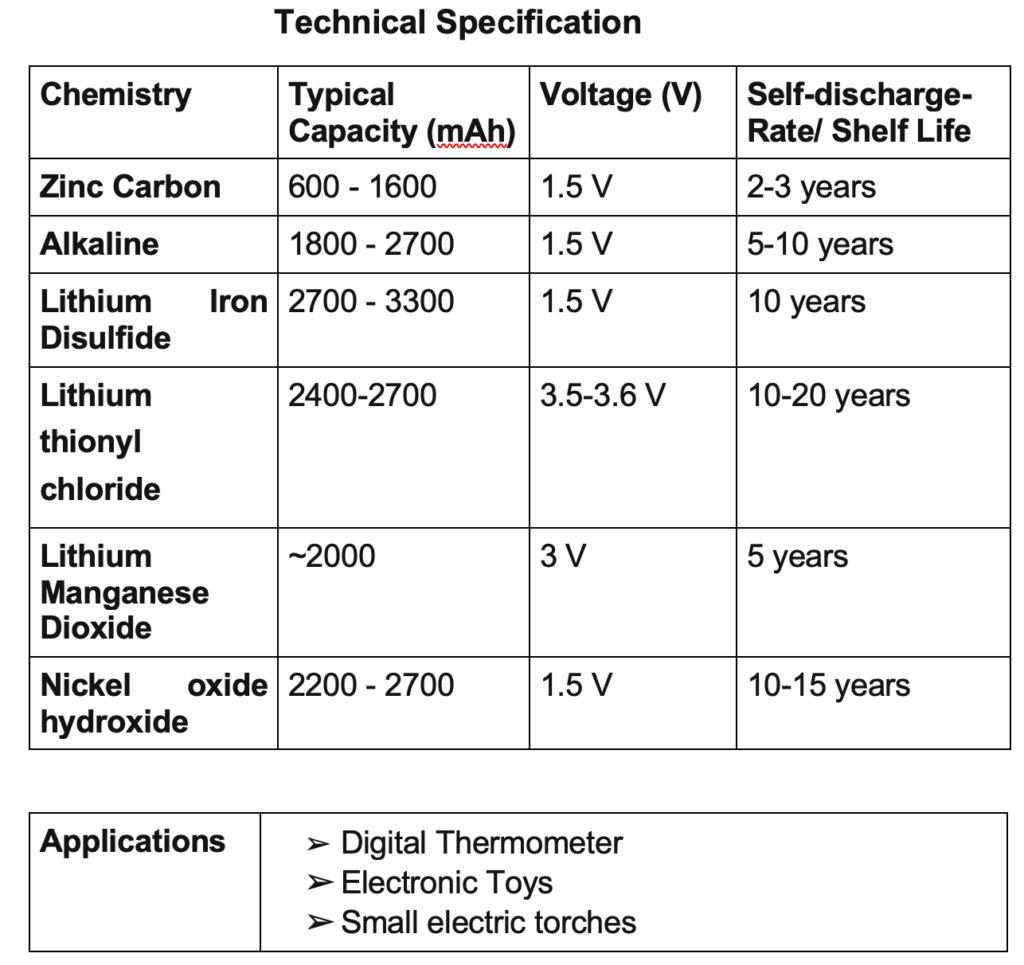
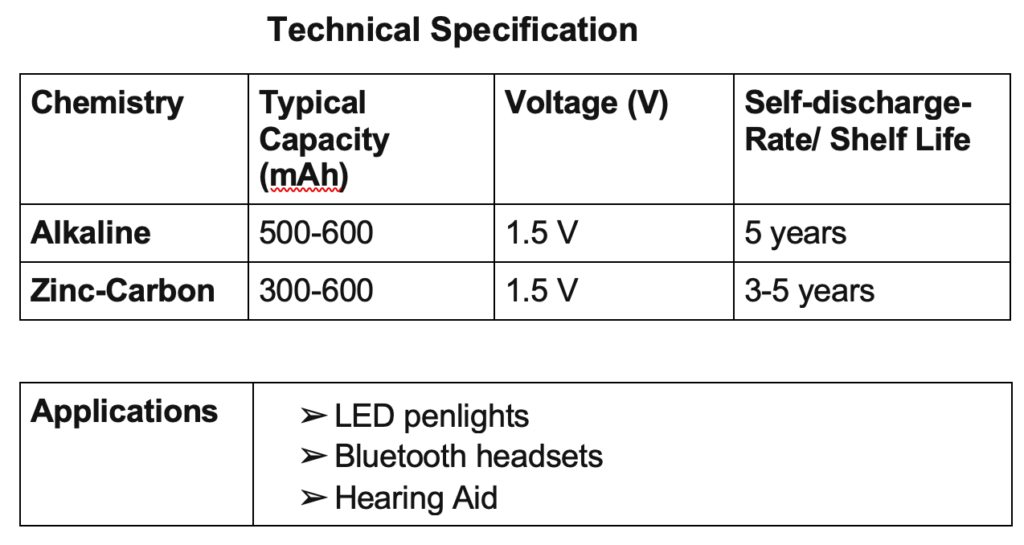
On the basis of nomenclature, types of primary batteries are the following.
AA Batteries
The AA battery is a standard size, single-cell cylindrical dry battery. It is an extremely common battery and is produced by many brands like Toshiba, Duracell, etc. It has a dimension of 50.5 mm in length and 14.5 mm in diameter.
AAA Batteries
AAA batteries are smaller in size as compared to AA but the capacity of an AA battery is much higher than AAA battery. It has a dimension of 44.5 mm in length and 10.5 mm in diameter.
AAAA Batteries
The AAAA battery weighs 43 percent less, 40 percent smaller, and is 20 percent thinner than the AAA battery. The AAAA battery is 42.5 mm long and 8.3 mm in diameter. These batteries are also classified as LR8D425 by IEC and 25A by ANSI/NEDA.
C Batteries
C batteries are a widely used type of dry cell battery that provides a reliable and long-lasting charge for devices with medium-to-high power consumption needs. It measures 500 mm x 26.3 mm.
D Batteries
D batteries are large cylindrical disposable cells that are used for applications drawing occasional power. These are larger than the C dry cells. The standard size of a D battery has an approximate length that ranges from 58.0 to 61.50 mm.
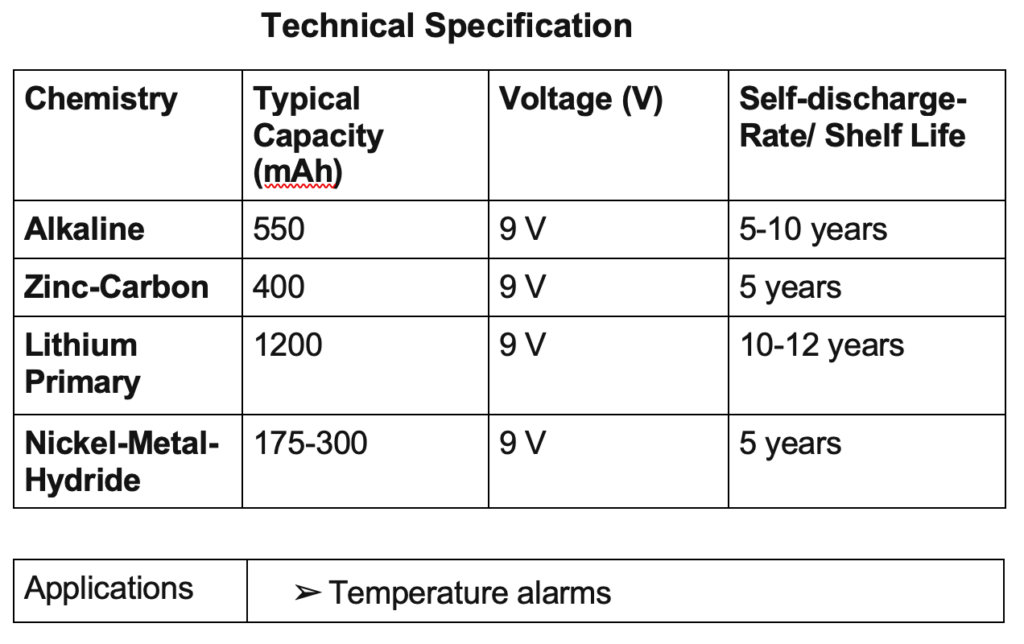
9 V batteries
This battery is rectangular in shape and has snap connectors at the top of the battery. The most common 9 V battery in this line is referred to as the PP3 battery.
CR123A batteries
This is a cylindrical cell battery which is widely used for many different applications, from medical devices to military-grade technology. They are commonly called the 123 battery. It is 34 mm in height and 17 mm in diameter.
23A batteries
An A23 battery is cylindrical and approximately two-thirds the length of an AAA battery. Its dimensions are 28.2 mm long and 10.0 mm in diameter. An A23 battery is basically an 8-cell device with a nominal voltage of 12 V.
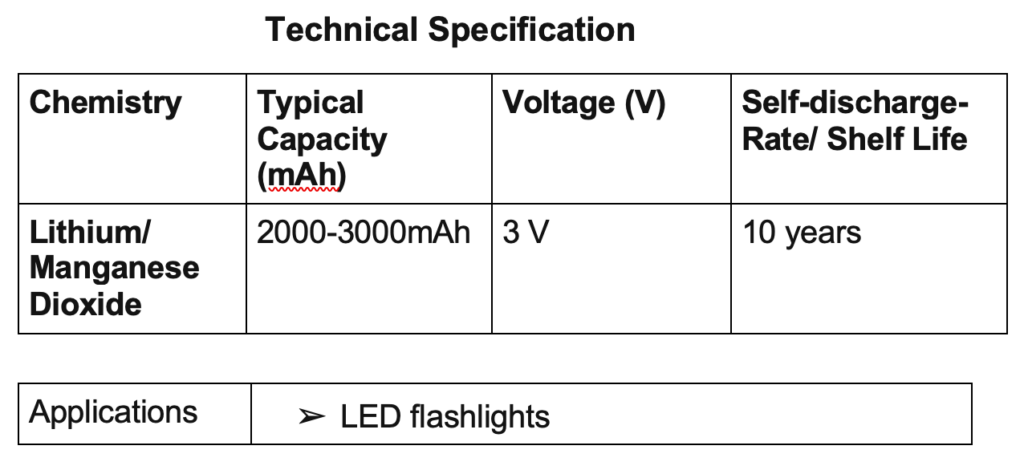
CR2032 batteries
This is a coin-cell battery — also called a button cell — which uses lithium chemistry. Compared to normal AA and AAA batteries, CR2032 lithium batteries are known to produce more efficient and stable power.
Secondary battery
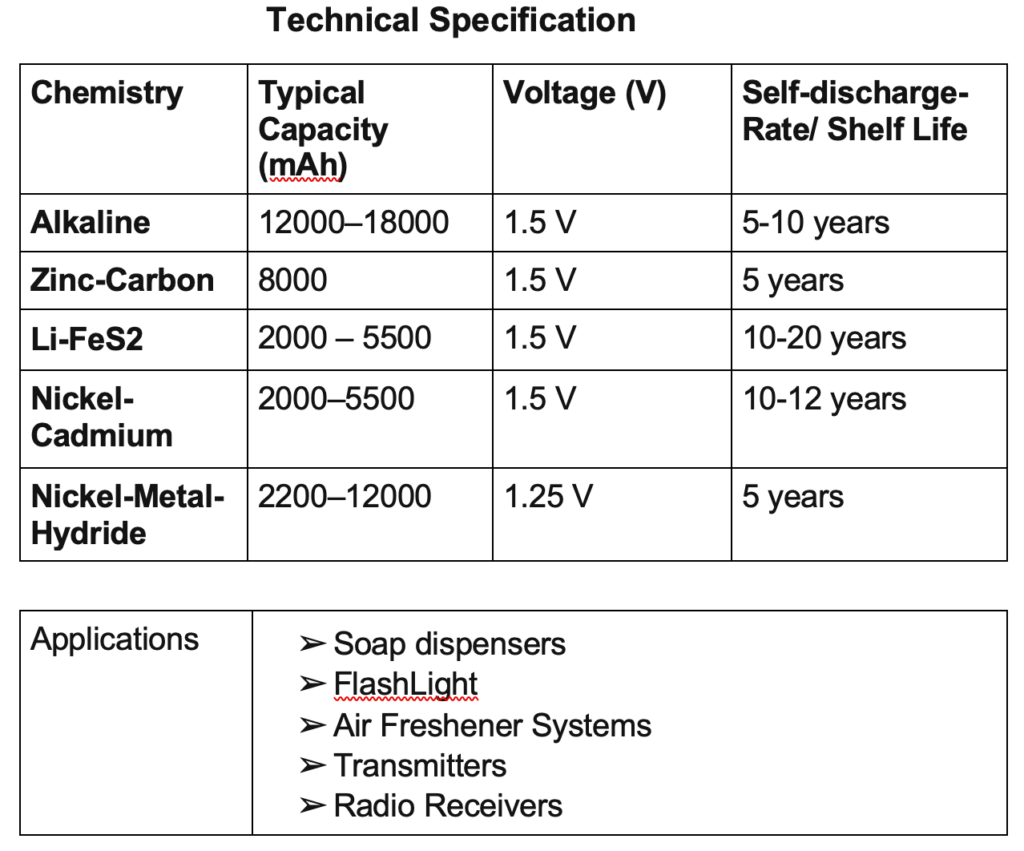
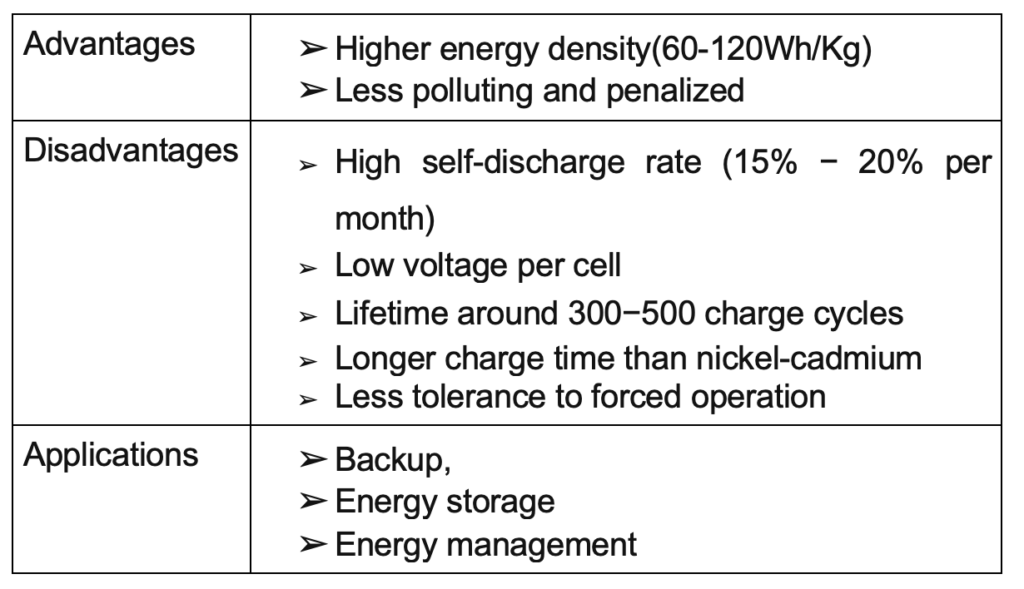
A secondary battery is electrically rechargeable. The most common secondary battery is the lead-acid battery used in automobiles. Examples are nickel-cadmium, nickel-metal hydride, and lithium batteries.
Lead Acid
This battery is composed of lead dioxide at the anode and a matrix or sponge of lead at the cathode. The electrolyte can be liquid (sulfuric acid and distilled water) or a paste or gel with a pressure-regulating valve.
Nickel Cadmium
Nickel-Cadmium is a battery composed of a cadmium anode and a nickel hydroxide cathode. The electrolyte is composed of potassium hydroxide. The charging technique for this battery is by constant current since there is no direct relationship between the voltage and the charge level.
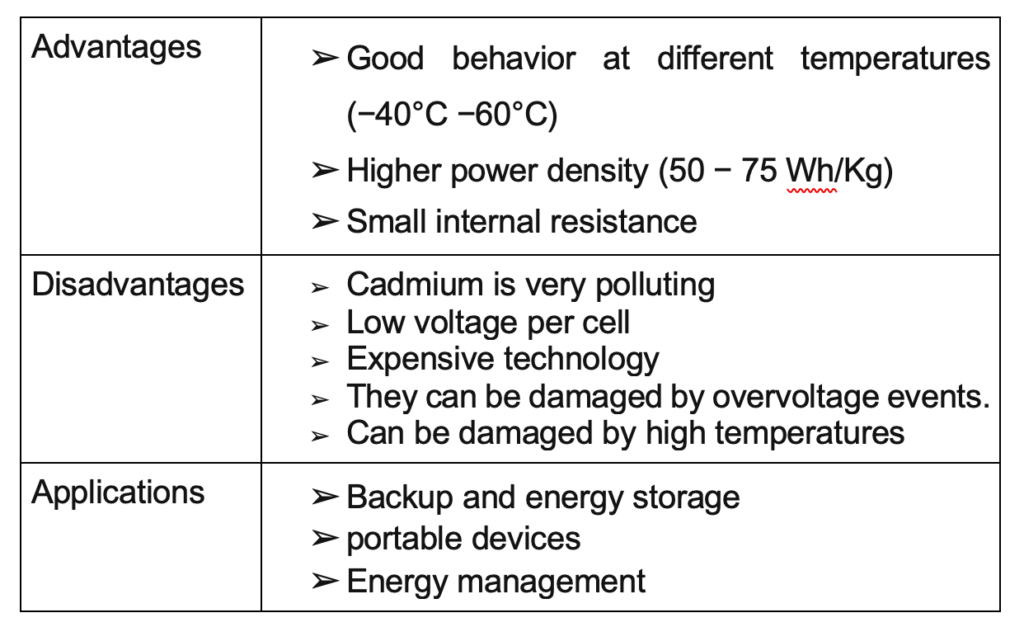 Nickel Metal Hydride
Nickel Metal Hydride
This is a battery composed of a nickel hydroxide anode and a metal hydride cathode. It is a modification or improvement of the nickel-cadmium battery. The main characteristics can be seen in table below.
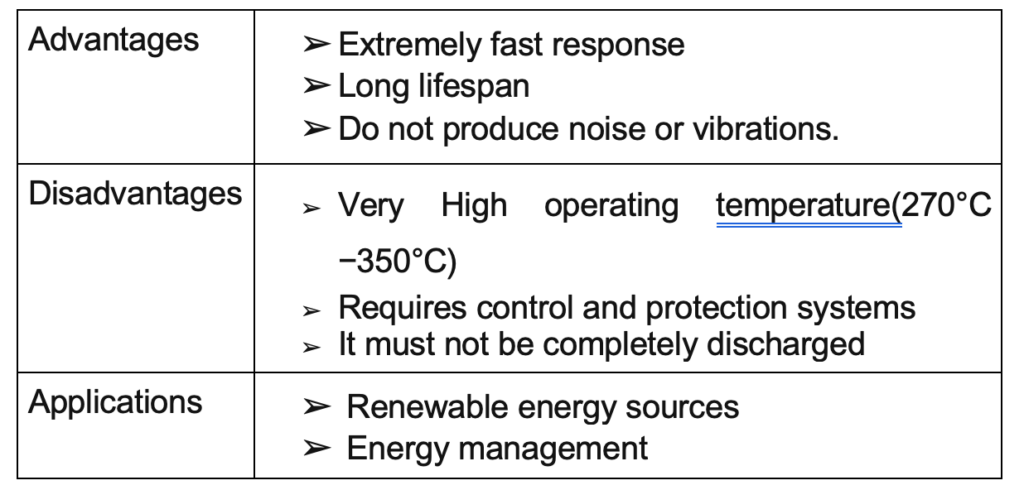
Sodium Sulfide
This type of battery with new technology has a sulfur anode and a sodium cathode. The electrolyte is a ceramic compound of aluminum oxide used as a separator and as an electrolyte.
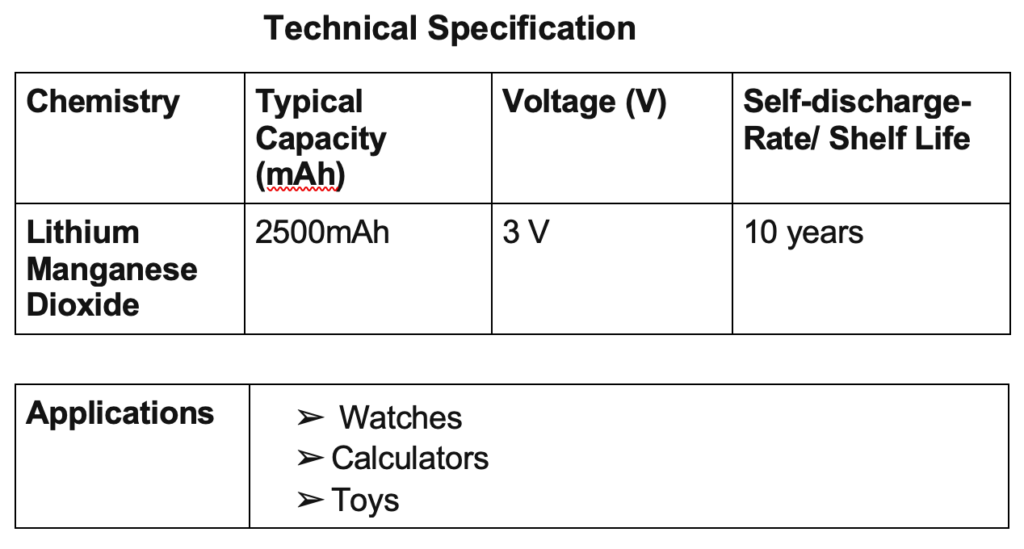
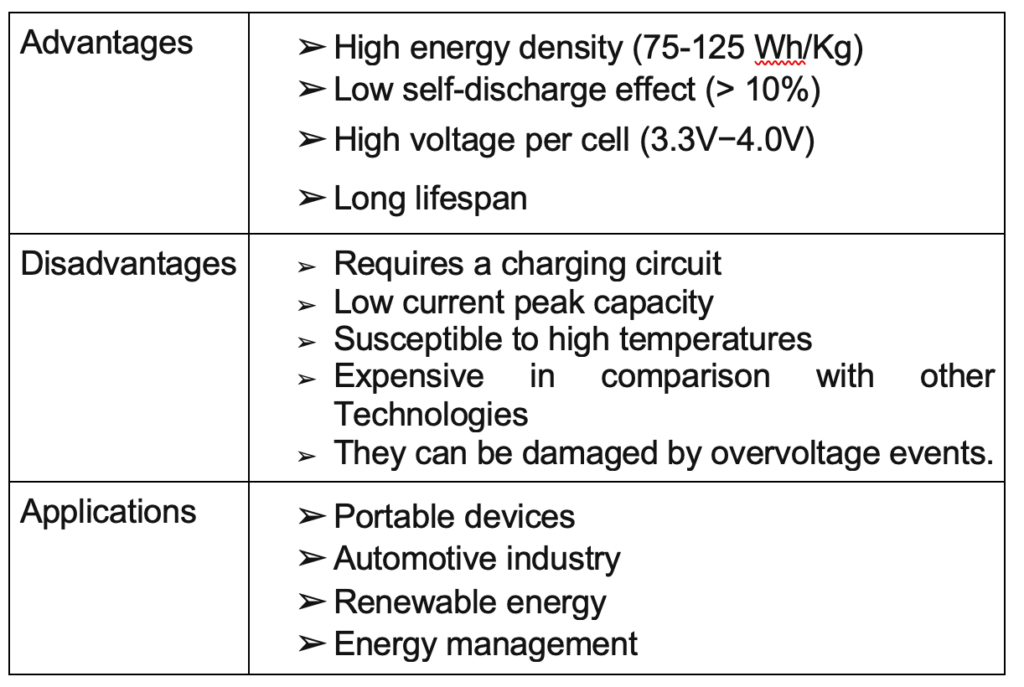
Lithium-Ion
Lithium-ion batteries are based on compounds with lithium in both electrodes, generally with graphite in the cathode and lithium in the anode. The charge-discharge process is based on the insertion-disinsertion of lithium ions, thus generating the conversion of chemical to electrical energy.
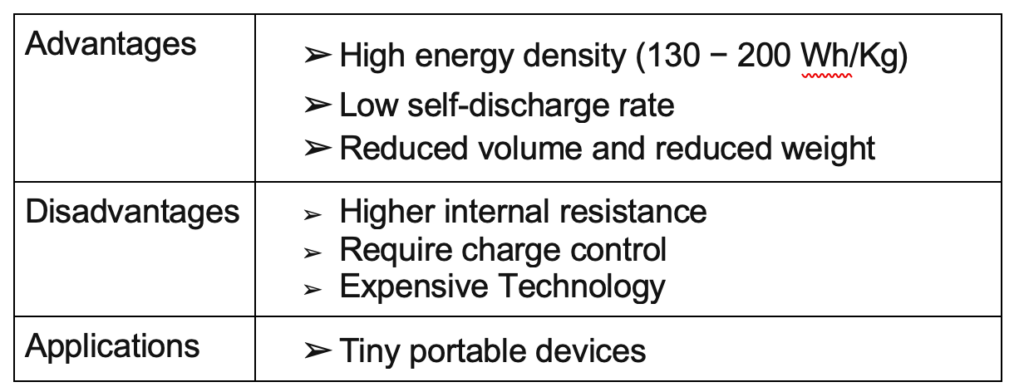
Lithium Polymer
The Li-Po battery is an improvement or modification to the lithium-ion battery, but its main characteristic is that the electrolyte is a solid polymer, allowing the creation of tiny batteries because the solid electrolyte uses less space than the liquid electrolyte.
Battery consideration
Let’s take an example. We have three cases:
1st case: Select a battery for the TV remote which should run for 1 year and consume very less power.
So, in this case, let’s say the IR LED in the remote should have a battery life of 1 year. Let’s assume that a person clicks a remote 100 times a day and every click takes 100 ms. The total time remote usage will be 10000 ms a day. Let’s take the battery of 100 mAh.
Battery Life = Battery Capacity( mAh )/ Load Current (mA) * 0.9
Battery Life = 100( mAh ) / 20(mA) * 0.9
Battery Life = 4.5 hours = 16,200,000 ms
Total number of days = 16,200,000 ms / 10000 ms = 1620 days
Number of years battery can run = 1620 / 365 = 4.4 years
Now, even a 100 mAh battery can last for more than four years. Therefore, we can consider AA, AAA, or AAAA batteries with a capacity lesser or equal to 100mAh.
2nd case: Select a battery for ECG coronary heart reveal so that it is able to be moved with the patient and is always ON for showing the patient’s vitals.
Let’s take an example of an IoT device that measures biosignals. It consumes 77mA at 3.8 V. It should run about six hours continuously before discharge.
Battery Life = Battery Capacity( mAh )/ Load Current (mA) * 0.9
Battery Life = 100( mAh ) / 20(mA) * 0.9
6 hours = Battery Capacity / 77mA * 0.9
Battery Capacity = 513 mAh
In this case, we will select a secondary batteries such as lithium-ion batteries and nickel-cadmium battery that can be used and whose capacity should be more than 513mAh.
3rd case: We want to select a battery that should run for a long time, about 10 years and consume moderate power.
Let’s take example of fire alarm whose standby load of 250mA, alarm load of 750mA and a standby period of 24 hours with 30 minutes in alarm condition:
Capacity = [(Standby load current x Time of Standby load current) + 1.75 x (Alarm Load Current x Time of Alarm Load Current)] x1.25
Capacity = 1.25((24 x 0.25) + 1.75(0.75 x 0.5))
= 1.25((6 + 0.66))
= 8.32 Ah
From this result, a minimum of 9Ah batteries would be required to maintain this system for the required period.
For this case, we will select primary batteries whose shelf life should be about 10-15 years and have a capacity of 9Ah. So we can choose any battery of lithium thionyl chloride composition whose power requirement meets our requirements.
Key considerations
We should always know the peak current (It is the maximum amount of current which output is capable of sourcing for brief periods of time) of the device as the average current consumption does not affect the battery. However, the peak current can have an adverse effect on the battery’s actual capacity, especially if there is not enough time between the periods of high current discharge (example: MCU wake up) to let the battery rest (example: MCU sleep) and recover.
To avoid these high current discharge periods, you should use a sufficiently large capacitor to supply the current to the device when the MCU is active. While the device is in sleep, the battery charges the capacitor, and when the device becomes active and requires a high discharge rate, this capacitor will provide current to the device. In this way, the battery would not experience high discharge rate periods and the battery efficiency can be improved.
In conclusion, if the power requirement is low or battery usage is infrequent over a long time, then a primary battery will be used. If the battery usage is frequent (if both primary and secondary batteries are capable of meeting the power requirement), the choice depends on user preference.


















Tell Us What You Think!