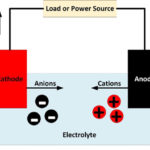
An anode is an electrode where oxidation reactions occur, involving atoms giving up electrons. The negatively charged free electrons then flow out of the negative terminal of the battery to produce an electrical current. It should be noted that conventionally, electricity is considered to flow in the opposite direction to the flow of electrons. Anions, negatively charged atoms which have excess electrons, flow towards an anode, flowing through the electrolyte in a battery or fuel cell. Cations, positively charged atoms which have lost electrons, are produced at the anode and flow towards the cathode.
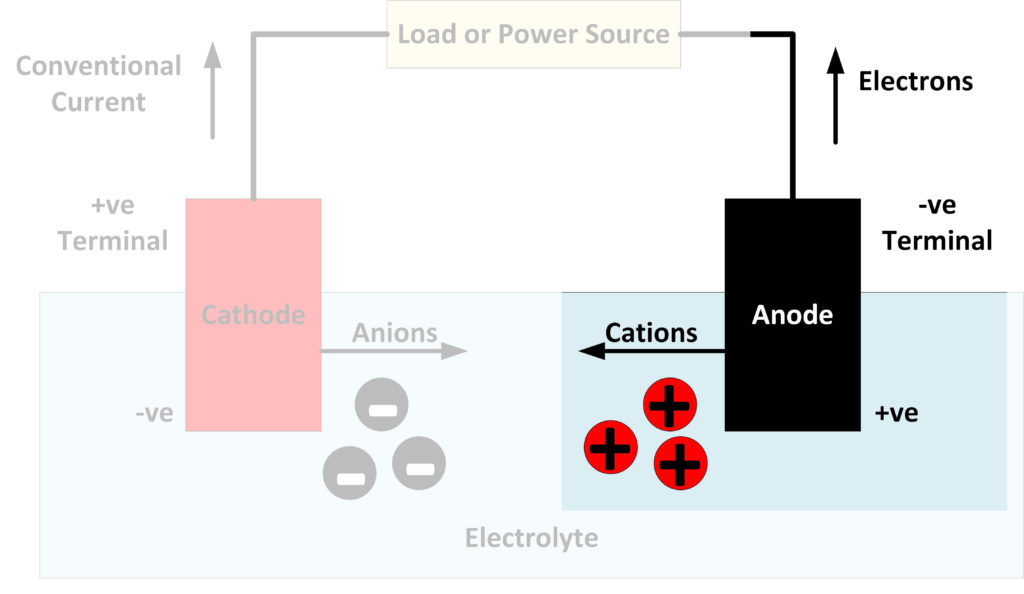 Although the external terminal connected to the anode is negative, the other end of the anode, which is in contact with the electrolyte is positive. This is what causes the negatively charged anions to flow towards it. It is at this end that the actual reactions take place, which produces the positively charged ions, known as cations, and the free electrons.
Although the external terminal connected to the anode is negative, the other end of the anode, which is in contact with the electrolyte is positive. This is what causes the negatively charged anions to flow towards it. It is at this end that the actual reactions take place, which produces the positively charged ions, known as cations, and the free electrons.
Anodes are found in batteries, fuel cells and other polarized electrical devices such as vacuum tubes. Although technically, an anode is defined as an electrode where oxidation occurs, with atoms giving up electrons, in a rechargeable battery this process reverses. It is, therefore, common to always refer to the anode as the electrode which acts as an anode during battery discharge. In reality, this electrode becomes a cathode during charging, when the flow of current reverses but is often continued to be referred to as the anode.
Many batteries use graphite as the anode material. Efforts to improve battery performance have, in recent years, tended to focus on improving cathode performance although there is now increased interest in improving anode performance. This has led to the use of lithium and silicon anodes, which could potentially increase the energy density of lithium-ion batteries by between 20 percent and 40 percent. Challenges include expansion leading to cracking as silicon anodes absorb electrons, and poor stability combined with high cost for lithium anodes. Aluminum has also been used as an anode material, with limited success in lithium-ion batteries although there have been significant recent advances in new aluminum-ion batteries.


Tell Us What You Think!