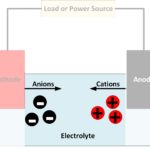
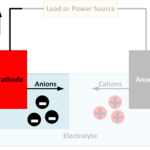
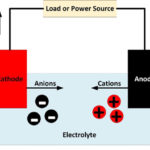
In general, an electrode is an electrical conductor which makes contact with a non-metallic part of a circuit. In a battery, the electrodes connect the battery terminals to the electrolyte. The electrode at the positive terminal is known as the cathode and the electrode at the negative terminal is known as the anode. Each electron is itself polarized, so that where they contact the electrolyte, the cathode is negatively charged and the anode is positively charged.
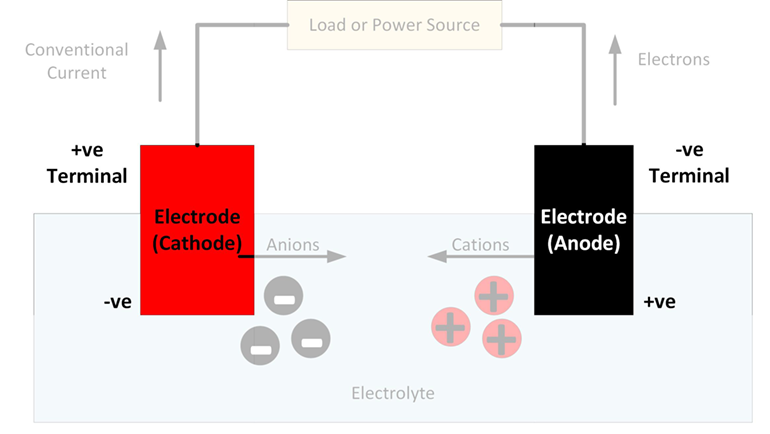 It is at the electrodes that the chemical reactions occur which cause an electrical current to flow during discharging or charging a battery. Reduction reactions take place at the cathode, with atoms gaining electrons to form negatively charged ions known as anions. Oxidation reactions take place at the anode, with atoms giving up electrons to form positively charged ions known as cations.
It is at the electrodes that the chemical reactions occur which cause an electrical current to flow during discharging or charging a battery. Reduction reactions take place at the cathode, with atoms gaining electrons to form negatively charged ions known as anions. Oxidation reactions take place at the anode, with atoms giving up electrons to form positively charged ions known as cations.
According to these technical definitions, the anode and cathode reverse depending on whether a battery is charging or discharging. However, when referring to the parts of a battery, it is conventional to name the electrodes according to their function during discharge. Therefore, the electrode that acts as a cathode during discharge is always referred to as the battery’s cathode, even through it actually becomes an anode during charging. Similarly, the electrode that acts as an anode during discharge, retains this name even though it actually becomes a cathode during charging.
As current flows through a battery, free electrons enter and leave the terminals at the ends of the electrodes contacting the electrical circuit. At the other end of the electrodes, clouds of ions form in the electrolyte.
Developments in commercial lithium-ion batteries have been driven by cathode chemistry. Specific lithium-ion batteries are, therefore, typically referred to by the cathode material. Examples include NMC (nickel-manganese-cobalt), LMO (lithium-manganese-oxide)and LFP (lithium-iron-phosphate cathode). As optimization of cathode materials matures, attention is now turning to anode materials, replacing graphite with alternatives such as silicone and lithium. Challenges include expansion cracking in silicone and a combination of cost and stability issues for lithium. Aluminum anodes have had very limited success in lithium-ion batteries and should not be confused with the completely new chemistry of aluminum-ion batteries.

very simple important information