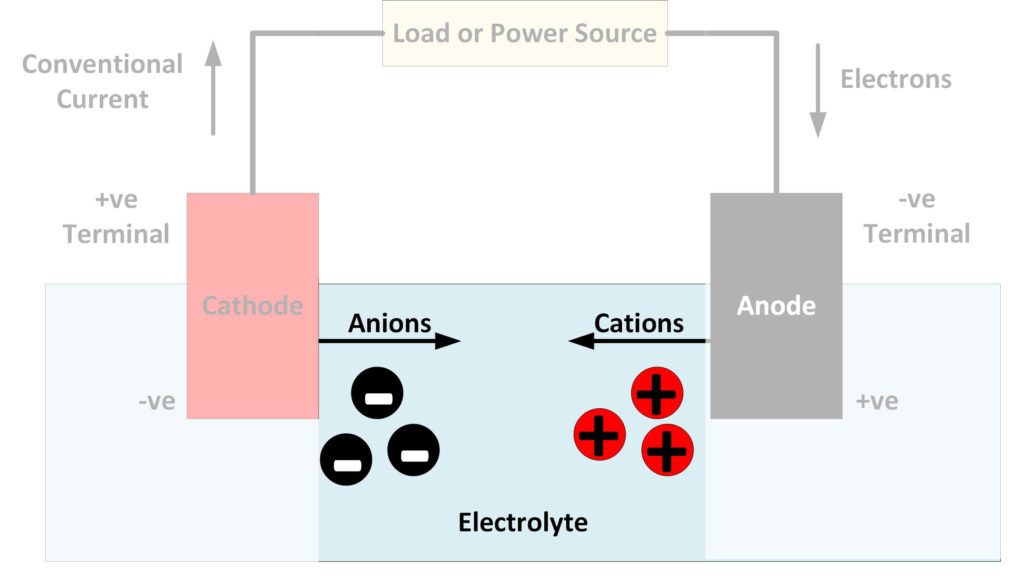
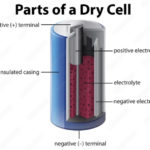
The electrolyte in a battery is the substance that allows electrical current to flow between the anode and the cathode. Electrolytes may be fluids or solids. Soluble salts, acids, and bases can generally act as electrolytes.
While current flows through a metallic conductor in the form of lone electrons, within an electrolyte current flows in the form of ions – atoms or molecules that carry charge due to the addition or subtraction of electrons. Electrons entering and leaving the battery terminals cause chemical reactions at the electrodes, which create clouds of these ions to form around them. Ions with opposite charge then flow towards each other.
Negatively charged ions, which are known as anions and have excess electrons, flow from the cathode to the anode. At the same time, positively charged ions, which are known as cations and have a deficit of electrons, flow from the anode to the cathode. Electrical charge is, therefore, flowing in both directions through the electrolyte.
Although most electrolytes are liquids, they do not always appear as a pool of liquid that is distinct from the electrodes.
 Examples of common types of batteries with their electrolytes:
Examples of common types of batteries with their electrolytes:
- A traditional lead-acid battery uses lead-based plates for both electrodes and a pool of liquid acid as the electrolyte.
- Alkaline single-use batteries use a zinc anode and a manganese-oxide cathode. The electrolyte is an alkaline solution of potassium hydroxide. Although it is a liquid, it does not appear as a distinct pool between the electrodes. Instead, the electrode materials are powders mixed with the electrolyte to form a paste. A thin ion-conducting layer separates the anode-electrolyte paste from the cathode-electrolyte paste.
- Zinc-carbon, single-use batteries have an electrolyte that is a wet paste of ammonium chloride and/or zinc chloride. This electrolyte paste is contained between the zinc case which acts as the anode, and a manganese oxide core which acts as the cathode. A graphite rod is used as the conductor through the cathode center, as the manganese oxide would rapidly corrode a metallic conductor.
- Lithium-ion batteries typically use a solution of lithium salt as the electrolyte. It is not present as a pool of liquid but rather is coated as a thin layer onto a separator sheet. The lithium metal oxide cathode is coated onto a thin copper foil, and the graphite anode is coated onto aluminum foil. These foils act as the current collectors which contact with the battery terminals, known as the tabs. The cell is then formed by layering these three sheets of material. They are often rolled up to form a cylindrical cell.


Tell Us What You Think!