Self-discharge refers to the declining state of charge of a battery while the battery is not being used. In most instances, self-discharge cannot be eliminated but needs to be managed. Too high a self-discharge rate can limit the potential applications for a battery. Depending on the battery chemistry and construction, there can be several causes of self-discharge.
This FAQ briefly compares the self-discharge rates of selected primary and secondary battery chemistries, reviews some of the challenges associated with measuring self-discharge, looks at chemistry-specific factors that affect self-discharge, how ultra-low self-discharge is achieved in certain primary lithium batteries, and closes with a look at recent research into how the type of tape used in Li-ion battery pack assembly has a significant impact on self-discharge rates.
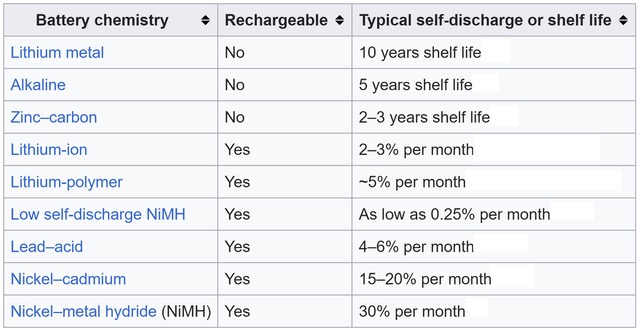
Table 1. Self-discharge rates can vary considerably for different battery chemistries (Table: Wikipedia).
Self-discharge can significantly limit the shelf life of batteries. The rate of self-discharge can be influenced by the ambient temperature, state of charge of the battery, battery construction, charging current, and other factors. Primary batteries tend to have lower self-discharge rates compared with rechargeable chemistries. But that’s not always the case; specially designed rechargeable nickel metal hydride (NiMH) batteries can have self-discharge rates as low as 0.25% per month (Table 1).
Measuring self-discharge
There’s not one method for measuring self-discharge. The method used depends on the battery chemistry and the level of accuracy required for the measurement. Measuring the open circuit voltage (Voc) is a common way to determine a battery’s state of charge and thereby measure self-discharge over time. But measuring Voc can be challenging.
For example, the discharge voltage curves of common rechargeable lithium chemistries like Li-manganese, Li-phosphate, and Li nickel manganese cobalt are very flat. The batteries need to be about 80% discharged before the voltage begins to drop significantly. Using Voc to measure the state of charge for those batteries is not useful since is can only indicate a full charge and about 80% discharged states. Fortunately, those chemistries have relatively low self-discharge rates compared with other rechargeable chemistries. For most Li rechargeable chemistries, coulomb counting during charging and discharging is generally used to determine the state of charge.
For lead acid batteries, measuring Voc can provide an easy first-order measurement of the SoC, and thereby a gauge of self-discharge. But lead-acid battery Voc is not that simple. The voltage rises with temperature and decreases with colder temperatures. In addition, calcium, a common additive in lead acid battery plates can increase the voltage by up to 8%. Increased levels of surface charge increase Voc immediately after charging, and a brief discharge can result in a measurable decrease in the voltage to a more realistic level. Absorbent glass mat (AGM) lead acids have a higher Voc than flooded types.
Finally, using a Voc measurement assumes that the circuit is open and the battery voltage is “floating” with no attached load. In modern systems, that’s not usually a valid assumption. Parasitic loads are often present for various housekeeping functions (like the digital clock in a car and other functions), and the battery is never completely disconnected.
Lead acid
In addition to the above factors, the self-discharge rate in lead acid batteries is dependent on the battery type and the ambient temperature. AGM and gel-type lead acids have a self-discharge rate of about 4% per month, while less expensive flooded batteries can have self-discharge rates of up to 8% per month.
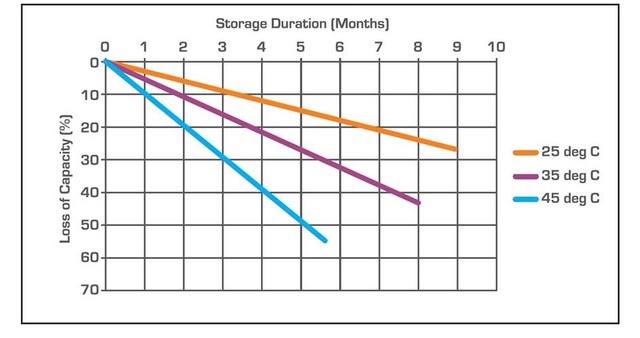
Figure 1. Self-discharge in lead acid batteries is highly temperature dependent (Image: Paypewatt).
The ambient temperature is probably the biggest factor affecting the self-discharge rate of lead-acid batteries. That can be important for applications like industrial uninterruptible power supplies (UPSs) or automobiles where the batteries can be subjected to high-temperature environments (Figure 1).
NiMH
The internal constructions of NiMH batteries have been optimized to deliver varying levels of capacity and self-discharge. High-capacity NiMh batteries use thin insulators that enable more active material to be included, increasing battery capacity by over 25% compared with standard designs. The thinner insulators, however, have shorter recharge lives and higher self-discharge.
A standard NiMH will retain 70% of its charge after 10 years in storage and can be recharged over 2,000 times. A high-capacity NiMH will lose up to 15% of its charge per year in storage and has a recharge life of 500 cycles. The high-capacity NiMH can also support higher drain rates compared with the standard design.
In addition, there’s a lightweight NiMH design that weighs about 30% less than a standard NiMH and is optimized for low-drain applications. It also has about 30% less capacity, can be recharged about 3,000 times, and has a self-discharge rate between the standard and high-capacity designs.
Li-ions
The breakdown of organic electrolytes is a common source of self-discharge in Li-ion batteries. Factors that impact Li-ion self-discharge include high temperatures and excessive humidity, both of which increase the rate of electrolyte breakdown. Excessive temperatures also cause the solid electrolyte interface (SEI) to deteriorate, further increasing self-discharge and loss of lithium. Moisture causes an electrolytic imbalance in the battery resulting in higher self-discharge rates.
In addition to electrolyte breakdown, the formation of micro-cracks in the separator contributes to self-discharge in Li-ion batteries. Microcracks can result from overcharging the battery and from the action of dissolved impurities in the electrolyte.
Self-discharge in Li-ion batteries cannot be eliminated, but it can be managed. Storing the batteries in cool but dry conditions will reduce the rate of electrolyte breakdown. The optimal storage temperature for many Li-ion chemistries is between 10 and 25 °C. Proper charge management to prevent overcharging reduces the formation of microcracks.
Lithium thionyl chloride
Primary Li chemistries like lithium thionyl chloride (LiSOCl2) can offer exceptionally low self-discharge rates that can result in multi-decade battery lifetimes. These batteries are available as spiral wound and bobbin constructions. Spiral wound cells can support high discharge rates. Bobbin cells have higher capacities and can have self-discharge rates under 1% per year enabling up to 40-year battery lives.
The passivation that occurs in LiSOCl2 batteries where a thin lithium chloride (LiCl) film forms on the Li anode is a major factor in the low self-discharge rates of these batteries. The passivation forms a barrier that limits the chemical reactions causing self-discharge. The passivation layer, however, also causes high initial resistance and a temporary drop in the cell voltage when the cell is initially discharged. As the cell discharges, the passivation layer dissipates, and the cell can deliver more current.
For low-power applications, some bobbin-style LiSOCl2 batteries can deliver a 40-year operating life. Though it’s not quite that simple. Self-discharge in these batteries is influenced by the current discharge capacity, the temperature and length of time the cell has been in storage, prior discharges of the cell, and the quality of the cell design.
The method of manufacturing and quality of the materials can have a significant impact on the self-discharge characteristics of LiSOCl2 batteries. High-quality LiSOCl2 batteries can retain up to 70% of their original capacity after 40 years. Low-quality LiSOCl2 batteries can have self-discharge rates of up to 3% per year and drop to 70% of initial capacity after only 10 years.
Tape affects Li-ion self-discharge
Researchers recently determined that the tape used to assemble Li-ion batteries can be a significant contributor to self-discharge. The polyethylene terephthalate (PET) tape that is used to hold the electrodes together experiences a chemical decomposition creating a compound that causes self-discharge.

Figure 2. At 70 °C, Li-ion cells turn dark red indicating the presence of a compound that causes self-discharge (Image: autoevolution).
The discovery was made when several cells were disassembled after being exposed to a range of temperatures. Using a common electrolyte, the cells were placed in ovens at 25, 40, 55, and 70 °C. The impact of temperature was visually evident. The cell kept at 25 °C kept its original clear color. The one at 40 °C was slightly discolored, the one at 55 °C was light brown, and the one held at 70 °C was dark red (Figure 2).
The PET tape decomposes at elevated temperatures and forms a redox shuttle molecule that travels back and forth between the positive and negative electrodes creating self-discharge. The redox shuttle molecule is always active in the background, even when the battery is in storage, and constantly discharges the battery. Replacing the PET with another type of tape is expected to reduce Li-ion battery self-discharge rates.
Summary
Self-discharge is an inherent characteristic of batteries. The rate of self-discharge differs among various battery chemistries. In addition, the quality of the materials used and the construction details of the battery can strongly influence the rate of self-discharge. Recently, it was found that the tape used to make Li-ion batteries can be a major contributor to self-discharge.
References
Comprehensive understanding of battery self discharge in lithium-ion batteries, Tycorun Energy
Dal researchers’ chance discovery could help extend battery life by replacing tape that causes self‑discharge, Dalhousie University
Self-discharge, Wikipedia
Understanding battery self-discharge, Tadiran Batteries
Why does a lead-acid battery self-discharge? Payperwatt




Tell Us What You Think!