The Leyden jar, invented by the Dutch physicist Pieter van Musschenbroek in 1746, preceded what we today call the battery. Originally, “battery” denoted several units connected in series or parallel. Thus, there could be a battery of Leyden jars.
Actually, the Leyden jar works on a principle completely different from that of a battery. It is a capacitor consisting of a glass jar lined on the inside and outside with electrically separate layers of foil. Originally the jar was filled with water because it was assumed that it contained the charge. Quite by accident, Luigi Galvani in 1780 constructed the first chemical-based energy cell. While dissecting a frog that happened to be hanging from a brass hook, he found that each time he touched the leg with his steel scalpel the leg moved. Galvani wrongly concluded that the leg itself was the energy source and named the force “animal electricity”.
A colleague, Allesandro Volta, realized the critical elements that made this primitive battery work were the two dissimilar metal electrodes and the saline electrolyte. He set about constructing what became known as a voltaic pile consisting of copper and zinc discs separated by layers of cloth saturated in saltwater. He eventually built an improved version with silver and zinc.
Today, of course, there are numerous battery chemistries whose qualities must be assessed to judge their practicality for specific applications. Two terms often come up in battery test equipment: battery testers and battery analyzers. Though the two terms are sometimes used interchangeably, they generally refer to two different types of equipment.
Battery analyzers were originally instruments used to restore nickel-cadmium batteries degraded by “memory” effects, a diminishment of capacity that arose when these batteries weren’t fully discharged. Today these instruments generally exercise battery packs of diverse chemistries, not just nickel-cadmium, to help decide when it’s time to retire packs that fall below some performance criteria.
Analyzers might perform operations such as discharging a battery to measure capacity, running the battery through charge, discharge, wait and repeat cycles; measurement of the internal battery resistance, discharging a battery at different current levels to simulate actual usage patterns, and rejuvinating a battery that has been over-charged.
On the other hand, battery test systems are generally found in research and development labs where they characterize battery properties. A test system might assess some of the same qualities checked via battery analyzers, along with several other parameters. Typical applications are life-cycle testing to simulate battery loading, as well as checking cell balance of multi-cell packs.
Battery test systems that work with huge battery banks, and significant amounts of power, often are regenerative. Instead of just dissipating battery energy in a static load, the system will send the battery output to some sort of synchronous inverter. The inverter converts the battery energy to ac power that is routed back to the utility grid as an energy saving measure.
Battery testers can run a variety of measurements. Among the most common are tests of actual battery capacity, battery cycle life testing, characterization of battery dc internal resistance, Hybrid Pulse Power Characterization (HPPC) tests (for batteries in hybrids and EVs), electric double-layer capacitor (EDLC) tests, lithium-ion capacitor (LIC) tests, and several others.
Battery testers generally run capacity tests at the final battery charge and discharge rate to gain a more accurate image of capacity than given by ordinary tests which take place at high charge and discharge rates. The same philosophy generally applies to battery cycle life tests.
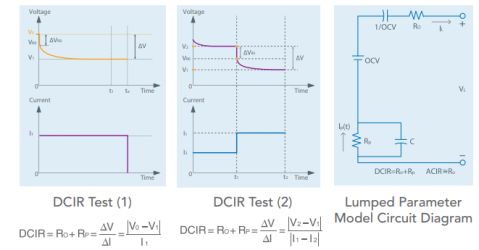
Because battery internal resistance can depend on loading current, battery testers may gauge dc internal resistance (DCIR) using more than one method. For example, they may use the voltage difference caused by the change of several different loading currents. HPPC tests were created by the US DoE to assess EV battery qualities such as depth of discharge, conductive resistance, and polarization resistance. Battery testers handling HPPC tests often do so without any manual intervention by the operator.
The IEC 62391 standard governs EDLC characterization and calls for charging the capacitor before testing the capacity and then discharging according to a prescribed curve. EDLC capacity gets calculated from the discharge energy and spacing time at prescribed points. Moreover, test conditions depend on whether the intended EDLC application is for energy storage, memory backup, transient power, or a power application. Similarly, dc internal resistance tests employ calculations made from the discharge curve, discharge time, and difference in discharge voltage.
There are similarities between tests of LICs and EDLCs. The applicable standard for LICs is IEC62813 which specifies the LIC be fully charged before testing. Calculations of LIC internal resistance also use points on the discharge curve in calculations of the resistance value.
Such tests are generally beyond what battery analyzers can do, but are well within the capability of sophisticated battery test systems.