When designing a battery into an EV, you must perform rigorous testing to understand a battery’s performance.
Electric vehicles (EVs) accounted for 13% of global vehicle sales in 2022 and are forecasted to reach 30% of global vehicle sales by 2030. The industry needs to continue working on making EVs affordable to support this transition from the internal combustion engine. This transition will require lots of batteries — better and cheaper ones, as they’re still the most expensive component in an electric car. According to McKinsey, the battery cells market is expected to grow by more than 20% per year until 2030, reaching $410 billion globally. That’s a 10x growth between 2020 and 2030.
Performance targets
Improving the batteries for EVs is key to improving vehicles’ economic, social, and environmental sustainability. The United States Advanced Battery Consortium LLC (USABC) has published guidelines to meet next-generation performance and cost goals for electric vehicle batteries.
As the automotive industry explores new battery chemistry and cell technologies, there are three big goals to increase EV adoption:
- Reduce the cost of electric vehicle batteries to less than $100/kWh—ultimately $75/kWh.
- Increase range of electric vehicles to 300 miles.
- Decrease charge time to 15 minutes or less.
Rigorous testing is the only way to ensure that every manufacturing cell will meet necessary performance standards. Many tests are conducted throughout the design, validation, and production process, but this article will focus on the critical few that help us understand the quality of the battery.
Open-circuit voltage (OCV)
Batteries store energy, which creates a voltage potential across its terminals. We use the energy in an electrical circuit. When nothing is connected to the battery, this potential is known as the open-circuit voltage (OCV). This value directly reflects the state of charge of the battery, a measure of how much energy the battery contains.
A battery’s OCV changes as it charges and discharges. Monitoring a battery’s state during cycling ensures that batteries are neither overcharged nor discharged. Batteries must be cycled multiple times during construction and OCV monitoring is part of the validation process and end application use. Larger battery packs, constructed of many cells, contain management chips that track cell and module OCV for status reporting.
When a battery is left to rest, disconnected from a load, there is still a small amount of current that flows internally. This is known as the self-discharge current. A battery cell’s OCV is relatively constant but will change by small amounts — in the tens to hundreds of microvolts — over the course of several weeks. This amount will be higher in lower quality batteries. OCV measurements let you monitor battery self-discharge and identify defective cells.
OCV is a relatively simple measurement. As Figure 1 shows, connect a digital multimeter (DMM) directly to the battery and measure DC voltage.
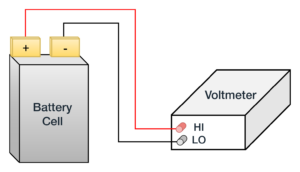
Figure 1. Open-circuit voltage Measurement on a Battery Cell requires a multimeter connected to the battery terminals.
For applications where the OCV only needs to be confirmed, any 6.5-digit DMM will do the job. In applications such as self-discharge testing, where it is necessary to detect small changes in the voltage, you’ll need an accurate higher resolution (7.5-digit) DMM.
For example, a good battery cell may decrease in charge by only a few millivolts to tens of millivolts over the course of four weeks. A failed or flawed battery cell might, however, see decreases in the hundreds of millivolts. On a smaller timescale, that could be down to microvolt levels from day to day. For an OCV measurement of 3.7 V on a cell, a typical 6.5-digit DMM can expect 142 µV of uncertainty in that measurement at 1 year calibration. A 7.5-digit DMM, however, can expect 63.8 µV of uncertainty for the equivalent measurement.
Internal impedance and resistance
It’s easy to think of a battery like a cup that can be filled with energy. When we would like some energy, we connect our circuit and let the energy flow out. This analogy doesn’t, however, account for internal impedance. A better analogy is that batteries are like water bottles. When a full water bottle is tipped over, the water cannot flow out freely because a nozzle — or even the neck if the bottle has one — restricts the flow. Similarly, a battery has internal impedance that restricts the flow of energy due to factors such as age, quality of materials, and imperfections in construction. This impedance is not purely resistive in nature, but has capacitive components as well, which presents a measurement challenge.
Just like OCV, the internal impedance reflects the quality of the battery and how well it performs over its lifespan. A battery that has higher internal impedance will be less efficient and prone to early failure. High internal impedance also generates excess heat during operation, which can become a safety issue if a battery goes into thermal runaway. Measuring internal impedance before use is a good way to identify cells that may be at risk of failure. For lithium-ion, a good battery cell can have an internal impedance of 100 mΩ, whereas a poor-quality or failed battery cells can be hundreds of milliohms. There are several ways to characterize internal impedance and each represents an aspect of the performance in a slightly different way.
Electrochemical impedance spectroscope (EIS)
The first technique is electrochemical impedance spectroscope (EIS). During this test, apply an AC signal to the battery (usually 100’s of milliamps, but several amps in some cases) over a wide spectrum of frequencies (0.5 Hz to over 100 kHz) and measure the battery’s response. This test can take several minutes to hours (lower frequency means longer test time) but gives the fullest picture of impedance behavior within the battery.
AC internal resistance (ACIR)
The most common method is known as AC internal resistance (ACIR). Because this an AC technique, it does characterize impedance. ACIR is a subset of the EIS process, taking a measurement at a single frequency, typically 1 kHz. This characterizes the small-signal behavior, which is a good indicator of battery quality and is much faster than the full EIS process. The time savings makes it a popular test in production, where every battery must pass specification.
DC internal resistance (DCIR)
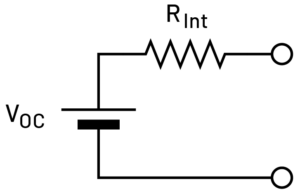
The final method is DC internal resistance (DCIR), which may be called pulsed characterization. In this method, only measure the resistive components, as we assume the battery to be represented by an ideal open-circuit voltage and a series resistance as shown in Figure 2.
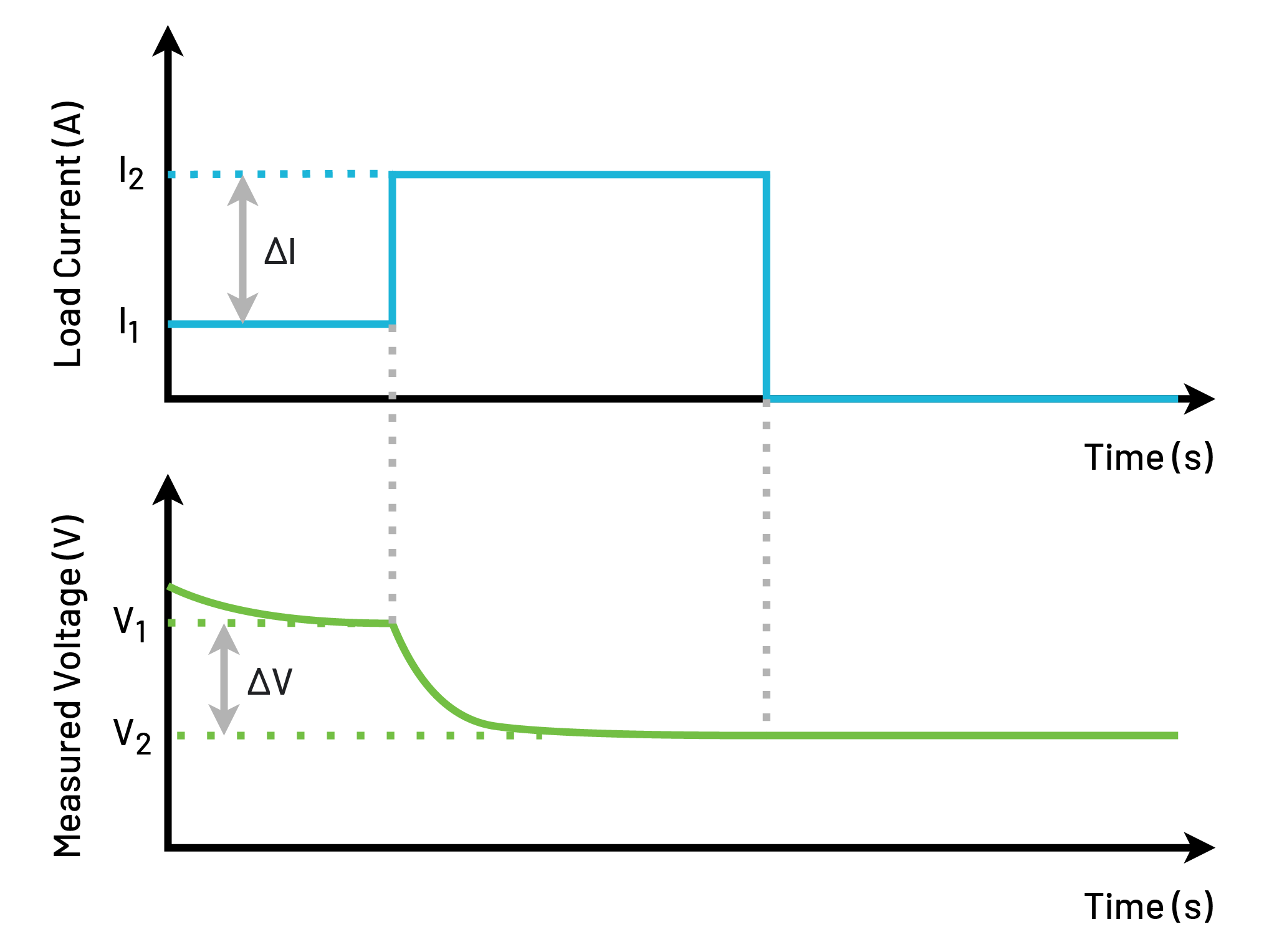
Apply DC current to the battery for a set amount of time. Measure the change in the battery’s voltage to calculate resistance. Figure 3 shows a graph that demonstrates this.
The currents used in the DCIR method are typically much larger (several amps) than the ACIR method (100 mA), making this a more realistic test in terms of use case as batteries are often exposed to sudden high current draw. The internal resistance of the battery is the biggest restrictor of a battery’s ability to output large current. Thus, it’s important to identify a battery that cannot perform under high current situations.
Test early and often
Many tests are conducted throughout the validation process, from the point of chemical and material fabrication (measuring isolation after electrolyte filling, cycling battery cells, short circuit conditions, etc.) to the point the cells are ready to pack and ship to their end application, such as grid energy storage, consumer electronics, and electric vehicles. The goal of validation and verification tests is always to identify defective cells before they move further in the process, wasting unnecessary material and time. Each test is necessary as there are many factors that can contribute to poor battery performance.
On the surface, OCV testing and internal impedance testing both have the same goal: to identify batteries that will not meet their performance expectations. Both are, however, needed as they each test unique factors. Open-circuit voltage looks at capacity and self-discharge, which can be caused by defects such as impurities in the separator or incorrect formation during manufacturing. These factors may not contribute to high impedance effects. Causes of high impedance such as a poor weld from electrode to tab can only be identified by direct impedance measurements.
For EV batteries, the goals set by USABC for optimizing safety, performance, and reliability should serve as a good guideline for current chemistries as well as emerging technologies beyond Lithium-ion. The key to effective performance characterization is to start with effective measurements that can identify failures. With the proper equipment and tests, manufacturers, researchers, designers, and even end users can get the full picture of their battery’s future performance.




Tell Us What You Think!