A group of researchers at the Hong Kong University of Science and Technology have come up with a coin cell that uses lead-free, bismuth-based, all-inorganic halide perovskite as an anode which also incorporates a photovoltaic cell to recharge itself.
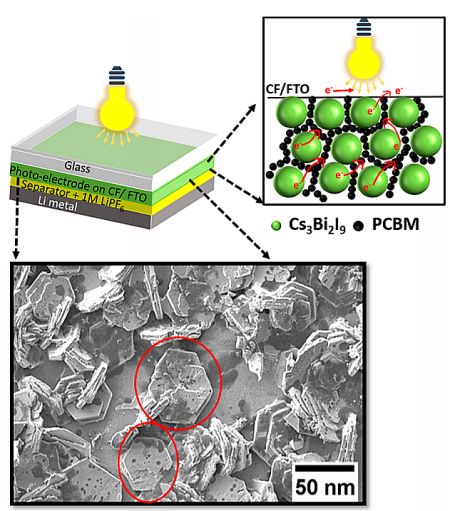
Top, a schematic representation of the internal structure of the photo-battery, and a microscopic view of the photoelectrode consisting of
Cs3Bi2I9 as the active material, PCBM as the conductive carbon, PVDF as the binder. Below, SEM image of Cs3Bi2I9 electrode (scale bar −50 nm).
Writing in the June 2021 journal Nano Letters, the group says they developed a perovskite that replaces lead with non-toxic bismuth (Bi), forming a strongly light-absorbing crystalline material that acts as a photoelectrode in a lithium-ion battery. The lead compounds used in existing perovskites are a potential source of lead poisoning. Moreover, the halide has higher stability in air.
The photo-battery consists of a layer-by-layer assembly of glass/current-collector/Cs3Bi2I9 photoelectrode/separator dipped in electrolyte with lithium metal as the cathode. The photoelectrode consists of Cs3Bi2I9 as the photoactive material mixed with PVDF (poly(vinylidene fluoride)) which helps bind the electrode materials and adhere to the current collector. The active layer is also doped with either carbon black as in standard (non-photo) coin-cells, or PCBM (phenyl-C61 butyric acid methyl ester) in the photo-battery cells, to improve transport of electrons to the current collector. The current collector can be made from copper, in the case of normal (non-photo) batteries, or by using transparent fluoride doped-tin oxide (FTO) and carbon felt (CF) for photo-batteries.
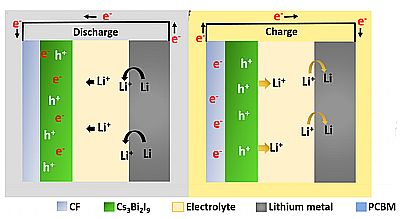
The discharge and photo-charge mechanism respectively of the Cs3Bi2I9 photoanode.
The researchers used three different current collectors–copper, fluorine-doped tin oxide (FTO), and carbon felt (CF)–to gage the electrode’s function as a normal coin cell and a transparent collector to realize a photo battery. They saw a photo conversion efficiency of ∼0.43% for the first discharge. Upon discharging under illumination, they saw a rise in capacity from 410 to 975 mA·h/g as compared to 372 mA·h/g obtained for commercial lithium-ion batteries. But there was an irreversible loss in capacity after the first discharge caused by the formation of a solid electrolyte interface and the conversion of some quantity of Bi3+ to Bi0, causing structural changes in the perovskite.
Researchers say the coin cell photo-battery can be discharged and then photo-recharged without any external current and then can power an external circuit for up to two hours before charging again.
To investigate the stability of the non-photo version of the battery, researchers cycled it 150 times at 100 mA/g with the first five cycles run at 50 mA/g. The Coulombic efficiency values rose from 86% to 96%; however, there was a gradual drop in the capacity values. They measured electrochemical impedance from 1 MHz to 0.01 Hz at 10 mV. They say the series resistance associated with liquid electrolyte didn’t change much after the first discharge and first charge cycles.
The researchers say they will experiment with different materials to boost the performance and efficiency of the photo-battery with an aim toward its commercialization.




Tell Us What You Think!