Diamagnetism, paramagnetism, ferromagnetism, ferrimagnetism, antiferromagnetism, and superparamagnetism are the six kinds of magnetism. This FAQ begins with a brief review of the basic sources of magnetism, considers the magnetic susceptibility of various materials, and then briefly presents the characteristics of the six types of magnetism.
The root cause of magnetism is the behavior of electrons, with temperature as a second-order consideration. The magnetic moments of electrons are the main source of magnetism. Atomic nuclei also have magnetic moments, but it’s generally several orders of magnitude smaller than that of electrons and negligible in the behavior of the overall material. Nuclear magnetic moments can be important in specialized contexts like magnetic resonance imaging (MRI) and nuclear magnetic resonance (NMR), but not for general magnetism.
Usually, the magnetic moments of the electrons in a material cancel out, and there’s no net magnetic field. That’s caused by factors like electrons combining into pairs with opposite magnetic moments due to the Pauli exclusion principle, electron subshells becoming filled, and there being zero net orbital motion. In addition, at a higher level, the various electrons in a material will have magnetic moments that are randomly arranged, and the material is not magnetic.
When the electrons align spontaneously or as a result of an external magnetic field, the electron magnetic moments and atomic moments can be lined up and produce a net magnetic field. How the electrons align determines any magnetic field’s type and strength (Figure 1). High temperatures result in large amounts of random thermal motion of the atoms, reducing the electrons’ ability to maintain any magnetic alignment. Many materials that exhibit magnetic properties at low temperatures can lose their magnetism at elevated temperatures.
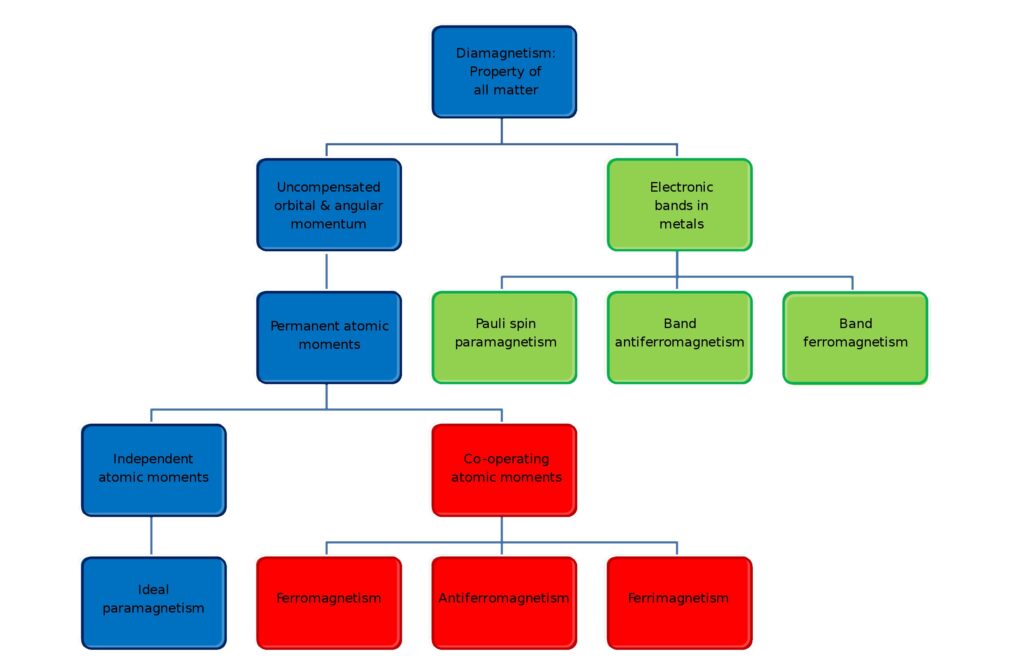
Figure 1: Diamagnetism appears in all materials, and the properties and behavior of electrons determine the type of magnetism exhibited by specific materials. (Image: Wikipedia)
Magnetic susceptibility
Susceptibility (χ) quantifies how much a material becomes magnetized due to an applied magnetic field. χ is the ratio of the magnetization (M) to the intensity of the applied magnetizing field (H), where M measures the magnetic moment per unit volume. At the simplest level:
- If χ is positive, the material is called paramagnetic, and the magnetic field in the material is strengthened by induced magnetization.
- If χ is negative, the material is diamagnetic, and the magnetic field in the material is weakened by the induced magnetization.
- Ferromagnetic, ferrimagnetic, and antiferromagnetic materials possess permanent magnetization without an external magnetic field and do not have a simple value for χ.
So-called ‘soft’ magnetic materials like iron and ferrites have high permeabilities and become magnetized relatively easily in the presence of an external field. On the other hand, “hard” magnetic materials like neodymium permanent magnets or air resist changing their magnetism in the presence of an external field (Table 1). Permeability can have different sources. In the case of neodymium magnets, the dipoles are not easily reoriented, hence the low permeability. On the other hand, air has relatively little mass and very few dipoles to reorient, resulting in low permeability.
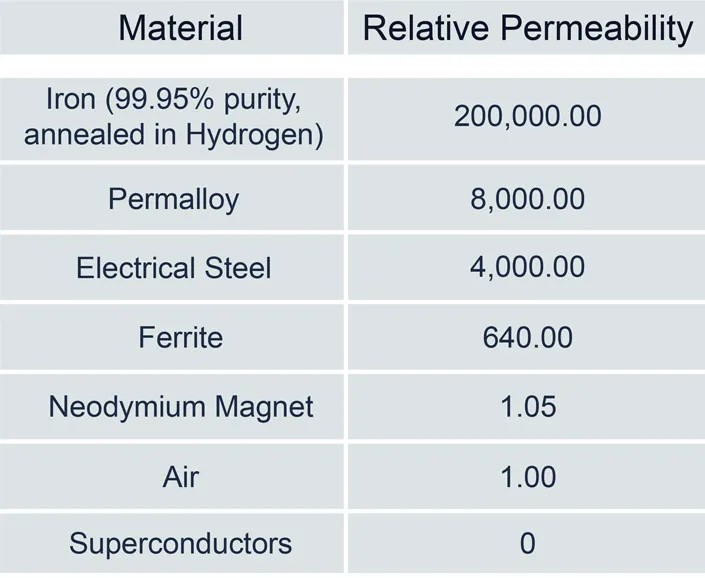
Table 1: The permeabilities of neodymium magnets and air are similar, but other properties result in different magnetic characteristics. (Table: Ideal Magnetic Solutions)
Diamagnetism
Diamagnetic materials create an induced field opposite to any applied external magnetic field and are repelled by the external field. Diamagnetism is present in all materials, and when it’s the only factor contributing to the magnetic properties, the material is called diamagnetic. Diamagnetism is a weak force and is easily overpowered in the presence of other types of magnetism, like paramagnetism or ferromagnetism. The atoms in a diamagnetic material have no permanent magnetic dipole moment. Diamagnetic materials have permeabilities less than that of a vacuum. According to classical physics, diamagnetism should be impossible and can only be explained using quantum-mechanical concepts.
Superconductors are a special case. While the diamagnetic effect is generally a very weak force, a superconductor is a strong diamagnet and completely repels any magnetic field from its interior, except for a thin surface effect, because of the Meissner effect. The Meissner effect occurs during the transition of a material to a superconducting state as it’s cooled below the material’s critical temperature. As a result of the Meissner effect, superconductors are called perfect diamagnets with χ = −1.
Paramagnetism
Paramagnetic materials form induced internal magnetic fields in the same direction as any applied magnetic field and are attached to the external field. Paramagnetism results from the presence of unpaired electrons. Since most atoms have some unpaired electrons, paramagnetic materials are common and include most chemical elements and a variety of compounds. These materials have a χ slightly over 1. On a basic level, if all the electrons in a material are paired, the material is probably diamagnetic; if there are unpaired electrons, it’s probably paramagnetic. There are a few exceptions, like copper.
Paramagnets don’t retain any magnetization when the external magnetic field is removed since the energy of thermal motion is sufficient to randomize the spin orientations. In an external magnetic field, induced magnetism in paramagnetic materials is small since only a small portion of the spins become oriented to the external field. The portion of spins that become oriented is linearly proportional to the field strength. That’s one of the distinctions between paramagnetic and ferromagnetic materials. In ferromagnetic materials, the portion of spin realignments is non-linear, resulting in a much stronger attractive force.
Ferromagnetism
Ferromagnetic materials have a large permeability (μ, the amount of magnetization that a material obtains in response to an applied magnetic field, measured in Henries per meter) and a significant coercivity (H, measured in Oersteds (Oe) or ampere meters and quantifies the ability of a ferromagnetic material to withstand an external magnetic field without becoming demagnetized). That combination of properties enables some ferromagnetic materials to form permanent magnets.
Ferromagnetic materials can be broadly subdivided into hard and soft materials. Hard materials have a higher H and tend to stay magnetized; soft materials have much lower values for H and are more easily demagnetized. A hysteresis curve can be used to visualize some of the differences between hard and soft materials. Figure 2 shows an example of hard and soft magnetic materials with significantly different values for H (where the curves cross the x-axis) but the same value for B, the magnetic flux density where the curves cross the y-axis.
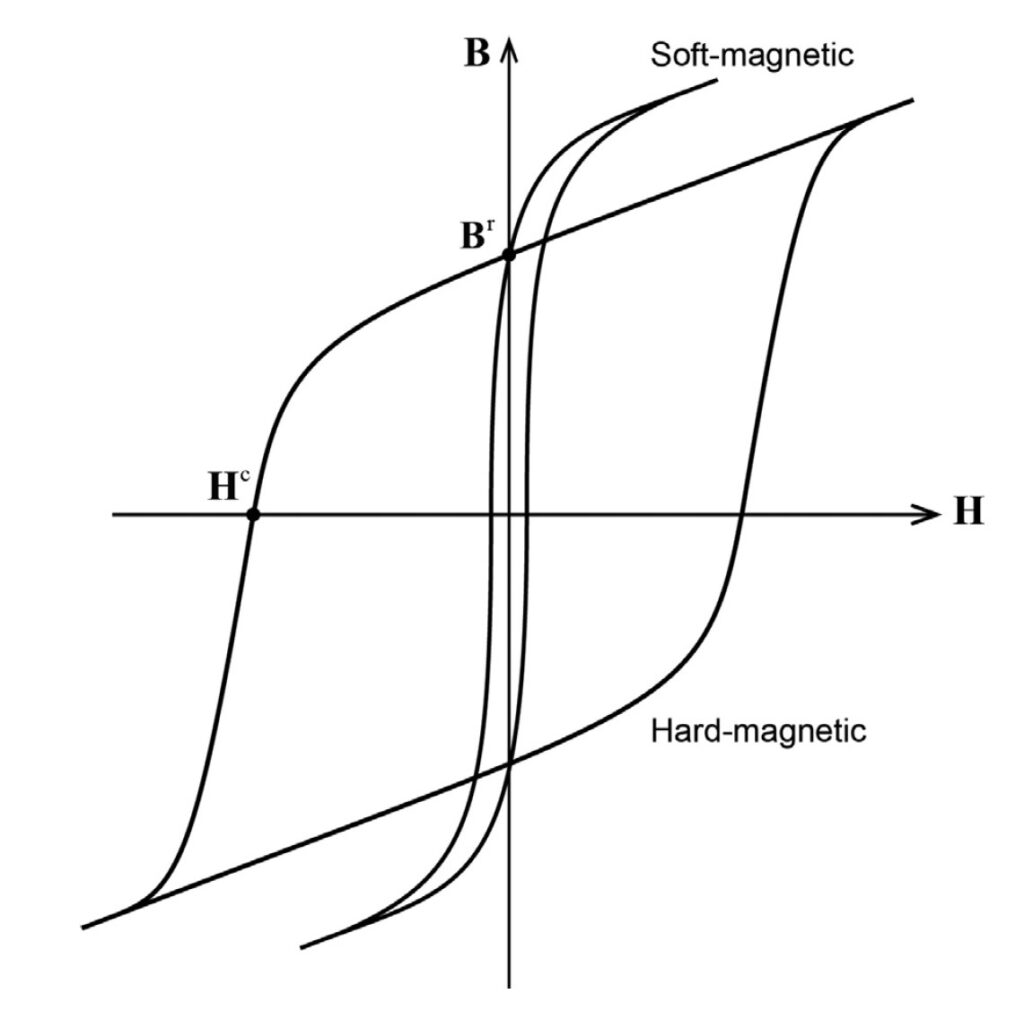
Figure 2: The hysteresis curves for soft ferromagnetic materials are narrower than those for hard materials. (Image: Journal of the Mechanics and Physics of Solids)
Ferrimagnetism
Ferrimagnetism has the same physical causes as ferromagnetism, both exhibit some similar magnetic properties, and the two can be easily confused. A ferrimagnetic material has atoms with opposing magnetic moments that are unequal in magnitude, so an inherent magnetism exists. The magnetism in a ferrimagnetic material also results from a combination of exchange interactions and dipole-dipole interactions caused by the Paul exclusion principle.
Like ferromagnets, ferrimagnets have a critical temperature, the Curie temperature, above which they become paramagnetic. At the Curie temperature, the thermal motion is sufficient so that the material can no longer maintain any inherent magnetism. Compared with ferromagnets, ferrimagnets have lower Curie temperatures.
Antiferromagnetism
Like ferromagnetism and ferrimagnetism, antiferromagnetism is a type of ordered magnetism. In antiferromagnetic materials, the magnetic moments of atoms or molecules align with neighboring spins on adjacent sublattices in a regular pattern pointing in opposite directions. Antiferromagnetic materials have small positive values for χ as the temperature decreases toward the Neel temperature (TN) of the material, χ increases and reaches a maximum at TN. The material is paramagnetic above TN and antiferromagnetic below TN.
Antiferromagnetic materials can couple to ferromagnets when a ferromagnetic film is grown on the antiferromagnetic material or annealed in an aligning magnetic field. When coupled, the antiferromagnetic material’s surface atoms align with the ferromagnetic material’s surface atoms. This enables the orientation of the ferromagnetic film to be ‘pinned,’ which enables the formation of magnetic sensors like the read heads in hard disk drives. The blocking temperature is the threshold at which an antiferromagnetic layer loses the ability to be ‘pinned’ to the magnetization direction of the adjacent ferromagnetic layer. The blocking temperature is usually below TN.
Superparamagnetism
Superparamagnetism is a form of magnetism that appears in small ferromagnetic or ferrimagnetic nanoparticles. It’s strongly size-dependent and only exists in nanocrystals below 3–50 nm, depending on the material. In a material in a superparamagnetic state, an external magnetic field can magnetize the nanoparticles, like in a paramagnet. But the magnetic susceptibility of superparamagnets is much larger than paramagnets.
A magnetic field is necessary for superparamagnetic materials to display magnetization, and these materials do not retain any magnetization once the magnetic field is removed. This reversible magnetization results in a different shape for the hysteresis curve (Figure 3).

Figure 3: The highly reversible nature of superparamagnetism results in a differently shaped hysteresis curve compared with ferromagnetic materials. (Image: European Journal of Nanomedicine)
Summary
The primary source of magnetism is the magnetic moments of electrons. In addition, temperature can have a significant impact on the magnetic properties of materials. The six types of magnetism are diamagnetism, paramagnetism, ferromagnetism, ferrimagnetism, antiferromagnetism, and superparamagnetism.
References
Basics of magnetic nanoparticles for their application in the field of magnetic fluid hyperthermia, European Journal of Nanomedicine
Diamagnetism, Advanced Magnetic Materials
Magnetic susceptibility, Wikipedia
Magnetism, Wikipedia
Mechanics of hard-magnetic and soft materials, Journal of the Mechanics and Physics of Solids
Shaping the Assembly of Superparamagnetic Nanoparticles, ACS Nano
Understanding magnetic permeability, Ideal Magnetic Solutions

Tell Us What You Think!