By Pamela Mansfield, JEOL USA
As the market for renewable energy sources and electric vehicles grows, the need for reliable, high-capacity energy storage is increasing too. Lithium-ion batteries (LIBs) fit the bill in many ways, but plenty of challenges remain ahead, such as understanding their microstructure. This article describes how scanning electron microscopy (SEM) can address these challenges and help LIB researchers and manufacturers refine and improve this energy-storage technology.
Recent global events have highlighted more than ever the need for reliable, high-capacity energy storage. It’s unsurprising then lithium-ion batteries (LIBs) continue to be favored for numerous applications, thanks to their combination of longevity, small size, and relatively low weight. However, the technology is far from optimized. Users continually demand improvements to LIBs — significantly increasing the energy density, improving the maximum number of charge cycles, and enhancing safety by reducing fire risk.

Figure 1: Scanning electron microscopy (SEM) image showing a cross-section through multiple layers of a LIB.
LIBs can contain as many as ten thin films of various materials (Figure 1). These can include ceramics, metallic foils, and polymers, with roles as the cathode, anode, insulation, current collector, and electrolyte. Such components may take the form of powders, sheets, or fluids and typically require inspection, both before and after assembly, as well as after repeated charge/discharge operations.
LIBs are complex, and analyzing them for research purposes can present challenges. Fortunately, microscopic and spectroscopic methods are designed to overcome these difficulties and simplify investigating LIBs at the microscale.
Understanding LIB materials at the microscale
Understanding the 3D microstructure of the individual layers – and the interfaces between them – is essential to get a complete picture of what’s happening inside a LIB. This requires high resolution and the ability to track minute changes on a material’s surface. But how can this be done?
The answer is scanning electron microscopy (SEM), which is ideally suited for detailed analysis of surface structure (Figure 2) and opens up possibilities to link the observed properties of the LIB with material composition and structure. For example, electrochemical behavior can be correlated to the physical status of the layers and the likely electrochemical processes involved — which may include ion relocation, lattice expansion or contraction, phase transitions, or surface reconstruction.
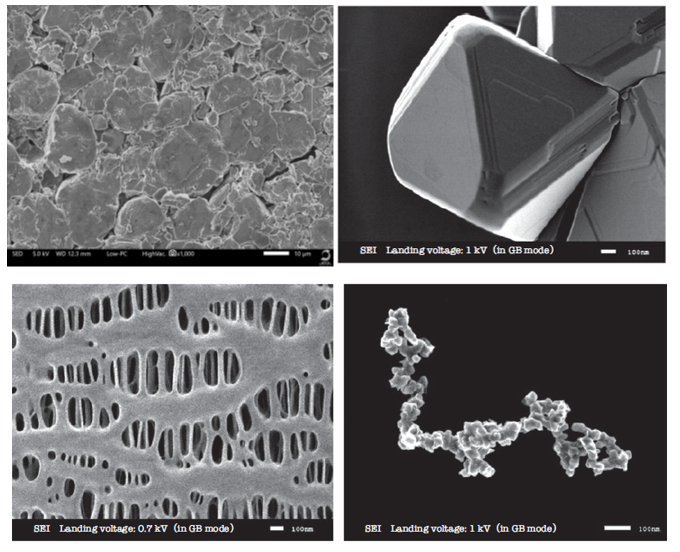
Figure 2: Examples of surface SEM images demonstrating the resolution achievable with SEM for various materials used in LIBs (clockwise from top left): anode active material; LiMn2O4 cathode active material; acetylene black conductive additive; polyethylene separator.
SEM instruments are widely used in the material sciences to visualize surfaces and cross-sections to understand grain structure and orientation on surfaces, determine grain boundary losses, and carry out defect analysis. With Energy Dispersive X-Ray Spectroscopy added onto an SEM, characteristic X-rays emitted by the sample can also be detected, meaning that elemental compositions can be determined (Figure 3).
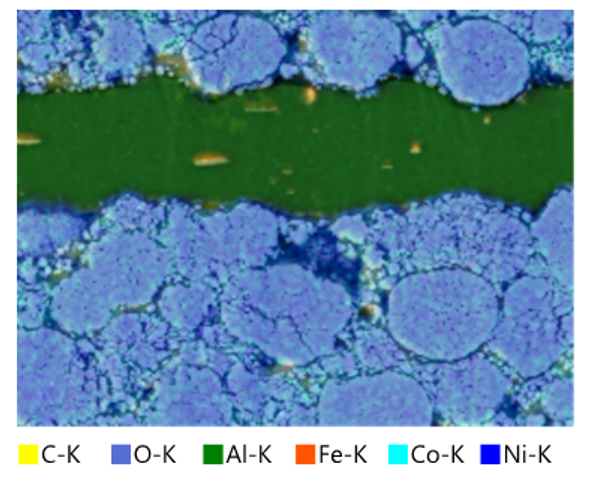
Figure 3: SEM-enabled X-ray elemental analysis of a LIB cathode, showing the distributions of elements.
In addition to helping researchers develop improved LIB formulations, SEM is also useful for quality control and troubleshooting – especially for detecting defects that reduce battery lifetime and reliability. Importantly for such investigations, the fast turnaround offered by modern SEM systems makes routine analysis of LIB cross-sections straightforward.
What is SEM?
Electron microscopy was invented in Germany in 1931 by Max Knoll and Ernst Ruska as a way to overcome the inherent resolution limit of visible-light microscopes (about 200 nm). The technique works by focusing a beam of electrons onto a sample using an electromagnet and detecting the resulting backscattered electrons and secondary electrons.
To generate an image of the surface, the electron beam needs to be scanned across the sample, and the first demonstration of this was in 1937. However, it was not until 1965 that the first commercial scanning electron microscope was launched. SEM instruments inherently offer a considerable depth of field. Thanks to accelerating voltage and electron optics improvements, resolution down to the sub-nanometer level can now be achieved. The technology has also been miniaturized, with systems now available in a convenient benchtop system.
Preparing the sample
Before imaging a cross-sectional sample using SEM, you need to prepare the sample properly. Traditionally, mechanical polishing was used, but this can introduce imperfections such as scratches and leave behind embedded pieces of polishing material, obscuring the original microstructure and compromising analysis. Broad-beam ion milling (Figure 4) using a tool known as a Cross Section Polisher resolves this problem by providing the clean surfaces essential for generating high-resolution images and producing accurate maps of chemical phases and elemental composition.
Materials used in LIBs don’t make things any easier because some of them can degrade upon exposure to air (Figure 5). To address this, air-isolated transfer vessels can prepare materials in an inert gas environment (such as a glove box) and then transfer them to the broad-beam ion milling system or directly to the SEM instrument. This avoids exposure to the air and so ensures a pristine sample.
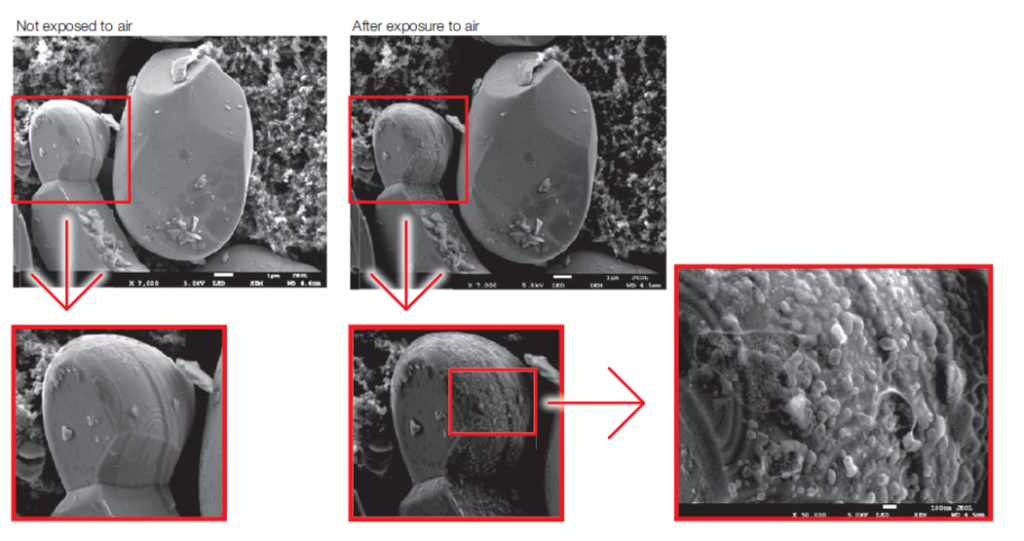
Figure 5: SEM images of LiCoO2 particles, visualized before and after air exposure, showing artifacts associated with exposure to atmospheric oxygen.
The future: understanding battery materials at the nanoscale
Looking forward, the need for improved LIB materials and designs will likely continue – meaning that manufacturers wanting to strengthen their position in the marketplace will need to use all the analytical tools available. One of these tools is SEM, which provides detailed images of specimen topology and microstructure and allows battery technologists to drive new product innovations and routinely confirm product quality.
Not only that, but the advent of benchtop systems is bringing this technology to a broader audience, allowing rapid analyses to be carried out on-site rather than in a dedicated analytical facility. As a result, integrating SEM technology into LIB research and manufacturing could help manufacturers push new boundaries in this field, allowing them to take their place at the forefront of the LIB industry.
For more information regarding SEM in LIB and energy research, please visit: https://www.jeolusa.com/APPLICATIONS/Application-List/Energy



Tell Us What You Think!