Lithium-ion batteries are a popular type of rechargeable battery which stores energy in lithium ions. They typically have a lithium metal oxide cathode and a graphite anode, separated by a thin layer of lithium salt solution acting as the electrolyte. Different battery chemistries use different cathode, anode, and electrolyte materials to change the battery’s performance. As well as different chemistries, there are also many different sizes of lithium-ion batteries. However, it is the battery chemistry that largely determines battery performance.

(Image: Panasonic)
Lithium-ion batteries have been developed and optimized for different applications. So far, most of this work has focused on the chemistry of the cathode. Batteries are, therefore, often named according to the cathode material. Important types of lithium-ion battery, categorized in this way, include:
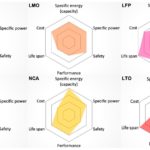
- Lithium nickel manganese cobalt oxide (NMC): Used in many high-performance vehicles due to very high energy density. Different quantities of the major constituents may be used. NMC-111, with equal parts of nickel manganese and cobalt, has been dominant, but NMC-811, with eight parts nickel to one part manganese and one part cobalt, is now seen as the future of NMC.
- Lithium nickel cobaltaluminum (NCA): Used by Tesla in some vehicles
- Lithium ferro phosphate (LFP): A lithium-iron compound which is the standard for low-cost electric vehicles in China. These batteries use cheaper and much more abundant materials, are safer, have higher power density, and much higher cycles lives (by two to four times). However, they are more expensive to fabricate and have lower energy density.
- Lithium manganese oxide (LMO): These are primarily used in power tools and electric bikes, although the first generation of Nissan Leaf used LMO. They are safe and don’t require any cobalt but have the lowest energy density.
Due to material shortages and serious ethical concerns in the supply chain, a lot of effort has gone into reducing the use of cobalt. LFP batteries are expected to become increasingly important as electric vehicle production is scaled.
State-of-the-art developments are now increasingly focusing on anode materials. It is believed that this might increase the energy density of lithium-ion batteries by a further 20% to 40%. Anode materials include:
- Graphite: Used for almost all production lithium-ion batteries. They are highly stable, producing batteries which last a long time. Although other materials can store more energy, none have been able to match graphite’s stability.
- Silicon: Silicon can store significantly more lithium ions than graphite. However, as silicon anodes absorb electrons, they expand, leading to cracking and, therefore, seriously impacting battery life. Recent research has attempted to create silicon nano-structures which resist cracking, but with little success.
- Aluminum: The use of aluminum cathodes in lithium-ion batteries has been attempted since the 1970s and should not be confused with aluminum-ion batteries, which are an entirely new battery type. Aluminum would theoretically make an excellent cathode as it forms lithium-rich intermetallic compounds giving a high energy storage capacity. It is also abundant, relatively cheap, and an excellent electrical conductor. However, stable anodes that provide long battery life have not been achieved, and research efforts have moved from aluminum to other materials.
- Lithium anodes are used in lithium metal batteries, single-use batteries which are distinctly different from rechargeable lithium-ion batteries. However, there is also some hope that lithium anodes may be another way to boost the performance of lithium-ion batteries.
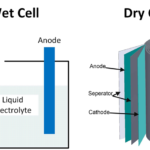
Solid state batteries change the chemistry of the third and final part of the battery, the electrolyte. They use the same anode and cathode materials. Conventional lithium-ion batteries all use a thin layer of lithium salt in an aqueous solution. This liquid electrolyte is contained as a thin film between the metallic foils of the anode and cathode. The electrolyte is a solid material in a solid state battery through which lithium ions can flow. Typically this is a crystalline glass compound containing lithium. It is hoped that solid state batteries will enable dramatic improvements in energy density, charging times, and safety. Although battery life remains relatively short, Toyota plans to start producing solid state batteries in 2022.


Tell Us What You Think!